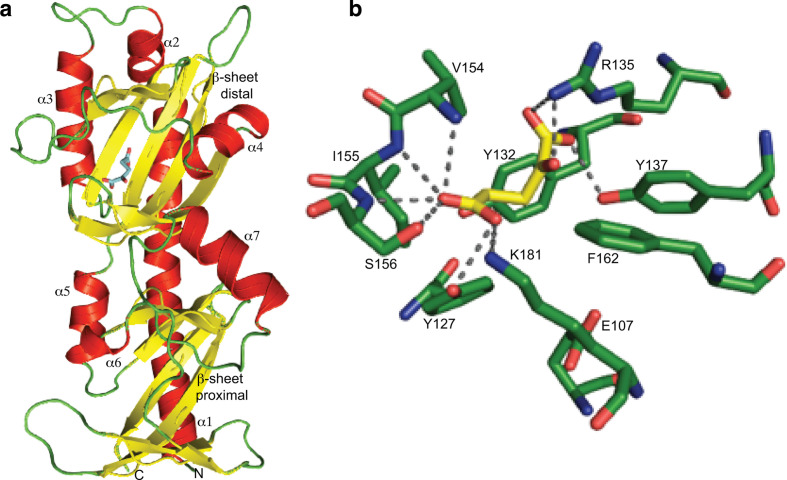
Fig. 3.
MifS sensor domain has an α-KG binding pocket. (a) Structural model of the MifS periplasmic sensor domain showing the two mixed α/β fold PDC domains, each containing a central β-sheet surrounded by three α-helices. The α-KG molecule is shown in blue bound to the distal PDC domain. The homology model of the MifS sensor domain was constructed using the SWISS-MODEL homology server [17]. (b) Model of the MifS binding pocket showing α-KG interacting with binding pocket residues via predicted hydrogen bonds to the side-chains and backbones of the binding pocket residues. α-KG was docked into the MifS sensor domain using AutoDock Vina [18] and protein–ligand hydrogen bonds were predicted using PyMOL [19].