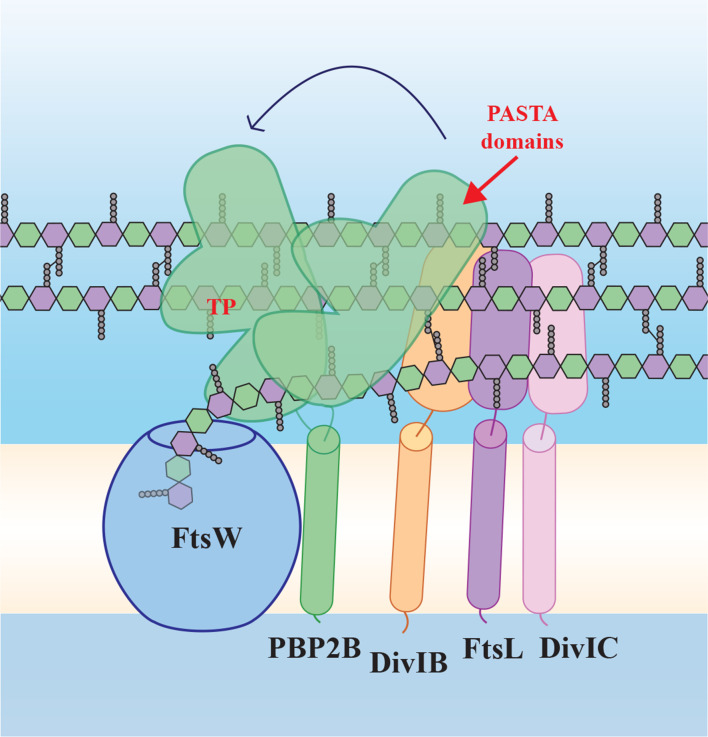
Fig. 1.
Schematic model of the ‘late division’ proteins based on the SEDS/bPBP structure [5] and our findings. FtsW and PBP2B form a SEDS/bPBP pair and interact via the transmembrane (TM) domain of PBP2B, but also via the extracytoplasmic part of PBP2B (containing the transpeptidase, TP, and PASTA domains), which has conformational flexibility. PBP2B interacts with DivIB and FtsL, which interacts with DivIC. According to our results, PBP2B PASTA domains play a role in strengthening the interaction between PBP2B and DivIB. FtsW polymerizes peptidoglycan building blocks, while PBP2B forms the cross-links between the strands. Yellow/white central band, membrane; peptidoglycan schematically drawn as hexagons (green, GlcNAc; purple, MurNAc) with attached pentapeptides.