Figure 1.
How Does AwareDX Determine Sex Risks?
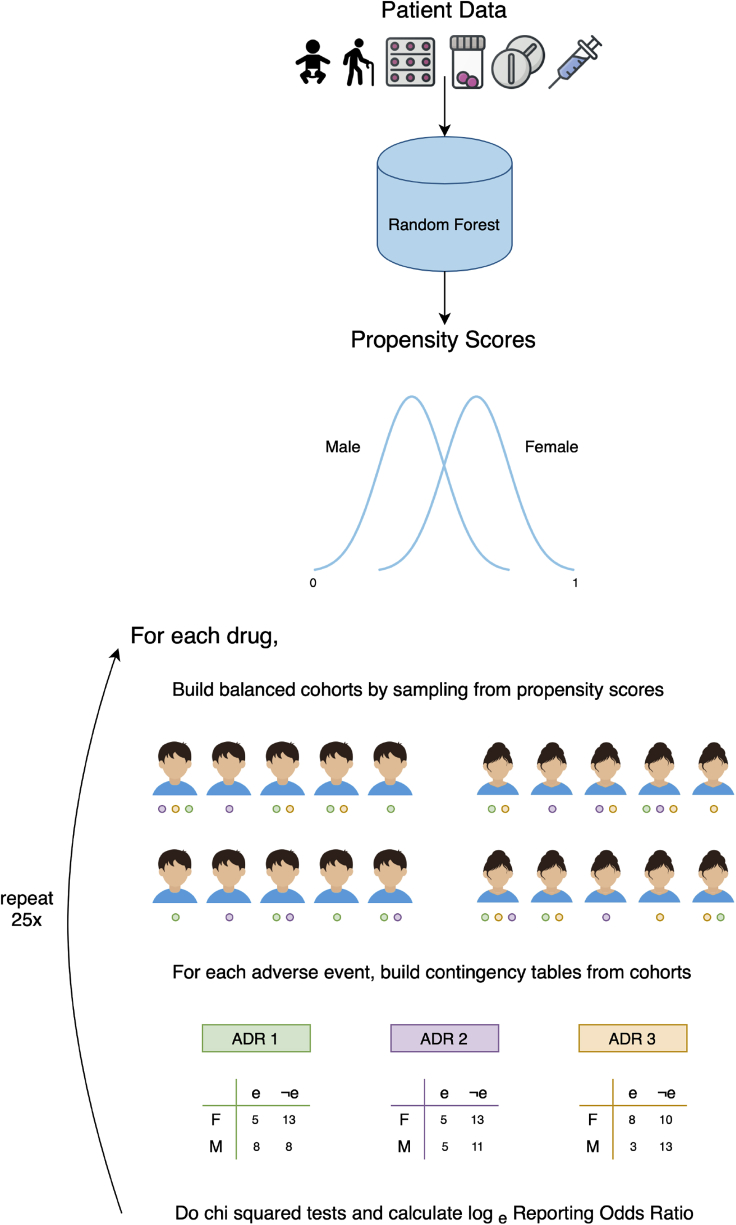
AwareDX evaluates sex risks in three steps: predicting propensity scores, building cohorts that mitigate bias, and doing disproportionality analysis. Starting at the top, for each patient, drug exposure, age, and co-medication features are curated from adverse event reports. A random forest model uses each patient's features to predict their propensity score, i.e., their likelihood of being female. These propensity scores are used to mitigate biases when evaluating sex risks. To analyze the sex risk of a drug and adverse event, the following steps are repeated for 25 iterations. The drug-exposed subpopulation is selected. Then propensity scores are used to build sex-balanced cohorts of that subpopulation. The patients within the cohorts have reported various adverse events, represented here by green, purple, and orange circles. For the adverse event of interest, a contingency matrix is constructed. A chi-square test supplies the P value for that iteration and the loge reporting odds ratio quantifies the sex risk. After 25 iterations, if all P values are significant, then the mean loge reporting odds ratio CI are used to quantify the sex risk. ADR, adverse drug event; F, female; M, male; e, has adverse event; e, does not have adverse event.