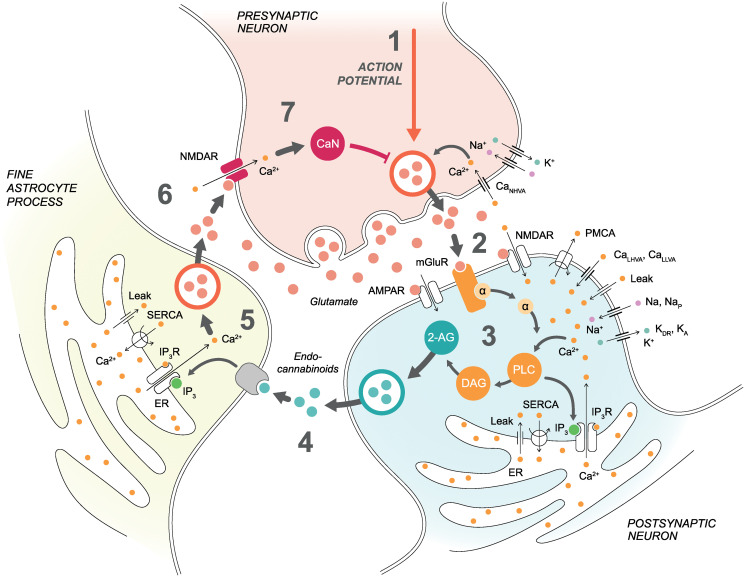
Fig 1. Schematic illustration of the synapse model.
Pre- and postsynaptic neurons and a fine astrocyte process are presented with key model components. (1) Presynaptic membrane potential depends on currents via CaNHVA, Na+, and K+ channels as well as via NMDARs. Presynaptic action potential and CaNHVA- and NMDAR-mediated Ca2+ concentrations together with the influence of CaN affect the vesicular release. (2) The released glutamate in the synaptic cleft activates postsynaptic mGluRs, NMDARs, and AMPARs in addition to presynaptic NMDARs. (3) Postsynaptic membrane potential in the soma depends on currents via Na+, NaP, and KDR channels, whereas postsynaptic membrane potential in the dendrite depends on currents via CaLHVA, CaLLVA, Na+, and KA channels as well as via NMDARs and AMPARs. The activation of postsynaptic mGluRs and NMDARs, together with the CaLHVA- and CaLLVA-mediated Ca2+ influx, triggers a G-protein signaling cascade where GαGTP dissociates from mGluR-bound Gβγ and activates PLC and production of DAG and IP3. Increases in Ca2+ and IP3 concentrations activate Ca2+ release via IP3Rs from the ER to the cytosol. On the other hand, PMCA and SERCA pumps transfer Ca2+ away from the cytosol and leak fluxes transfer Ca2+ back to the cytosol. The production of DAG leads to a production of endocannabinoid 2-AG. (4) Endocannabinoid 2-AG released from the postsynaptic neuron binds to the astrocytic CB1Rs and triggers Ca2+ signaling in the astrocyte. We modeled this step by directly modifying astrocytic IP3 concentration based on the postsynaptic 2-AG concentration. (5) Astrocytic IP3 and Ca2+ activate similar ER-related events as in the postsynaptic neuron. Astrocytic Ca2+ increase then induces glutamate exocytosis to the extrasynaptic space. (6) Glutamate in the extrasynaptic space and the spillover of glutamate from the synaptic cleft activate presynaptic NMDARs. (7) Presynaptic NMDAR-mediated Ca2+ concentration activates CaN, and CaN has an effect on vesicular release together with presynaptic action potential and CaNHVA-mediated Ca2+ concentration.