Figure 3. Applications of GECAs and potential solutions for wireless optogenetics.
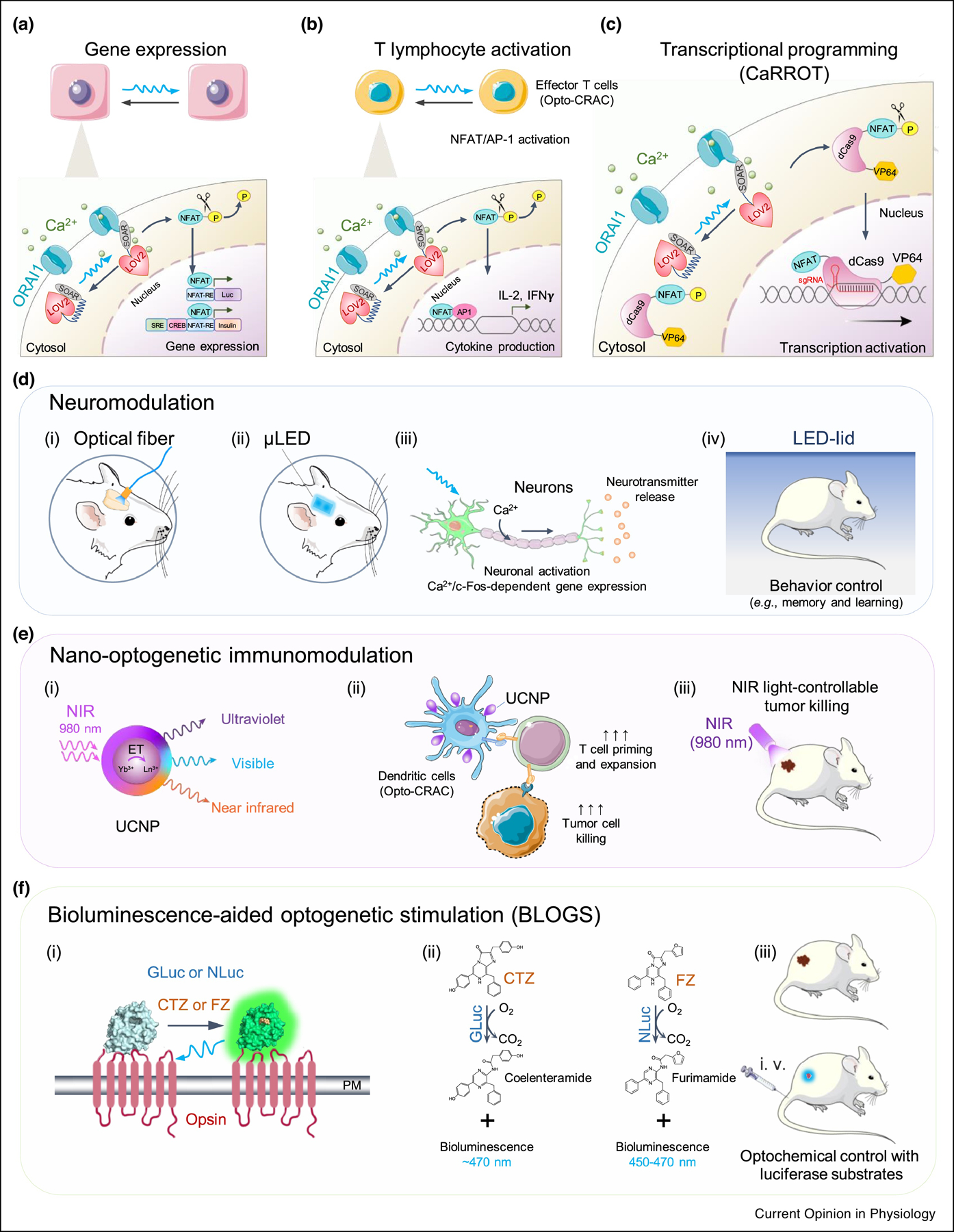
a. GECAs (e.g., Opto-CRAC) utilized to control Ca2+-dependent gene expression using synthetic response elements (RE) derived from various Ca2+-responsive transcription factors. NFAT, nuclear factor of activated-T cells; CREB: cAMP response element-binding domain; SRE, serum response element.
b. GECAs used to control Ca2+/NFAT-driven cytokine production in T cells in the presence of co-stimulatory signals to activate activator protein 1 (AP-1).
c. Coupling GECAs with CRISPRa to enable light-tunable transcriptional programming. Upon blue light illumination, Opto-CRAC1 induces cytosolic Ca2+ influx, which in turn activates CaM/calcineurin to dephosphorylate the N-terminal non-DNA binding fragment of NFAT (NFAT1–460) and eventually drive the nuclear entry of the fusion protein (NFAT1–460-dCas9-VP64) – a calcium-responsive transcriptional reprogramming tool (designated as CaRROT). In the presence of small guide RNAs (sgRNAs), CaRROT can be precisely targeted to the promoter regions of any desired genomic loci to photo-tune the expression of endogenous genes.
d. Optogenetic neuromodulation with GECAs (e.g., OptoSTIM1 or monSTIM1). Blue light can be delivered into a specific brain region via implantation of optical fiber (i) or μLED (ii). Photoactivatable Ca2+ influx can lead to induction of Ca2+-responsive gene expression (e.g., c-Fos) in both neurons and astrocytes in deep-brain regions such as hippocampal and thalamic regions (iii), thereby modulating the behavior (e.g., social fear learning) in awake mice (iv).
e. A nano-optogenetic platform for wireless optogenetic immunomodulation in a mouse model of melanoma. (i) schematic illustration of the core/shell structure of lanthanide doped upconversion nanoparticles (UCNPs), which act as nanoilluminator to emit visible light upon near-infrared irradiation (NIR); (ii-iii) NIR light-controllable activation of Ca2+ influx in dendritic cells (DCs) expressing Opto-CRAC1 could boost antigen presentation and DC maturation to more efficiently prime T cells in the draining lymph nodes, thereby facilitating dendritic cell-based immunomodulatory therapy against melanoma.
f. Bioluminescence-aided optogenetic stimulation (BLOGS). (i) Cartoon illustrating the photoactivation of a chimeric luminopsin (LMO) receptor comprising a microbial opsin and Gaussia luciferase (GLuc) or NanoLuc luciferase (NLuc) in the presence of their cognate substrates coelenterazine (CTZ) or furimazine (FZ). (ii) Photochemical reactions catalyzed by GLuc or NLuc. (iii) Schematic depiction of wireless optogenetics using BLOGS in mice administered with luciferase substrates.
