Abstract
This review details the current understanding of the mechanism of action and corneal effects of mitomycin C (MMC) for prophylactic prevention of stromal fibrosis after photorefractive keratectomy (PRK), and includes discussion of available information on dosage and exposure time recommended for MMC during PRK. MMC is an alkylating agent, with DNA-crosslinking activity, that inhibits DNA replication and cellular proliferation. It acts as a pro-drug and requires reduction in the tissue to be converted to an active agent capable of DNA alkylation. Although MMC augments the early keratocyte apoptosis wave in the anterior corneal stroma, its most important effect responsible for inhibition of fibrosis in surface ablation procedures such as PRK is via the inhibition of mitosis of myofibroblast precursor cells during the first few weeks after PRK. MMC use is especially useful when treating eyes with higher levels of myopia (≥ approximately 6 D), which have shown higher risk of developing fibrosis (also clinically termed late haze). Studies have supported the use of MMC at a concentration of 0.02%, rather than lower doses (such as 0.01% or 0.002%), for optimal reduction of fibrosis after PRK. Exposure times for 0.02% MMC longer than 40 seconds may be beneficial for moderate to high myopia (≥ 6D), but shorter exposures times appear to be equally effective for lower levels of myopia. Although MMC treatment may also be beneficial in preventing fibrosis after PRK treatments for hyperopia and astigmatism, more studies are needed. Thus, despite the clinical use of MMC after PRK for nearly twenty years—with limited evidence of harmful effects in the cornea—many decades of experience will be needed to exclude late long-term effects that could be noted after MMC treatment.
1. Introduction
Several long-term studies have supported the efficacy, predictability, stability, and safety of photorefractive keratectomy (PRK) (Hashemi et al., 2017; Hersh et al., 1997; Rajan et al., 2004; Shalchi et al., 2015). Limitations of PRK have included regression of refractive correction and the development of vision-limiting fibrosis (referred to clinically as late haze) caused by an exacerbated corneal wound healing response where large numbers of myofibroblasts are generated (Kaiserman et al., 2017; Marino et al., 2016; Torricelli et al., 2016; Wilson, 2020).
Corneal fibrosis can lead to glare, halos, irregular astigmatism, loss of uncorrected visual acuity (UCVA), loss of best-corrected visual acuity (BCVA), and regression of the effect of PRK surgery. Each of these complications can prolong visual recovery and rarely even yield permanently diminished vision requiring lamellar corneal transplantation or penetrating keratoplasty. Since the incidence of fibrosis increases with increasing levels of attempted correction with PRK, the possibility it could develop has tended to decrease the level of dioptric treatment (Hersh et al., 1997; Torricelli et al., 2016).
The incidence of corneal fibrosis after PRK was higher with first-generation excimer lasers, such as the Summit Technology (Waltham, MA) excimer laser or Autonomous (Alcon, Ft. Worth, TX) excimer laser, likely because of unsatisfactory excimer laser-beam profiles that produced surface irregularity, which impaired regeneration of the epithelial basement membrane (EBM) (Vestergaard et al., 2013; Netto et al., 2006a). Advances in excimer laser technology have greatly reduced, but not completely eliminated, corneal fibrosis after PRK—even when MMC is used at the time of surgery. Risk factors for the development of corneal fibrosis include hyperopia, high myopia, high astigmatism, environmental ultraviolet light exposure, prior corneal surgery, and possibly genetic influences, whereas older age may have a protective effect (Ang et al., 2016; Corbett et al., 1996; Kaiserman et al., 2017; Salomao and Wilson, 2009; Thomas et al., 2008).
Talamo et al. (1991) were the first to suggest the use of MMC eye drops as a modulator of corneal healing in rabbits. Schipper et al. (1997) first applied MMC to the residual stroma bed in rabbits. Majmudar and collaborators (2000) were the first to apply MMC in human corneas to treat corneal haze, while Carones et al. (2002) were the first to study prophylactic MMC to prevent corneal fibrosis after corneal surface correction of high myopia. This anti-proliferative medication has subsequently been shown to prevent and treat corneal fibrosis after PRK, decrease refractive regression, promote greater accuracy of refractive surgical correction, and, therefore, allow safer treatment of higher ranges of myopia (Bedei et al., 2006; Carones et al., 2002; Chen et al., 2011; Majmudar et al., 2015; Sorkin et al., 2019). There, however, remain some concerns about potential long-term adverse effects of MMC on corneal stromal cells and corneal endothelial cells that lead some surgeons to continue to take a more cautious approach to using the medication.
This review will highlight our currently understanding of the mechanism of action of MMC in favorably regulating corneal wound healing responses. It also outlines the currently available data on drug dosing, exposure time, and eye safety.
2. Molecular basis of mitomycin C alkylation, DNA cross-linking activity and its cellular effects
Mitomycin C (C15H18N4O5) (Fig. 1), belongs to the mitomycin family—a group of potent antibiotics discovered in the 1950s by Japanese microbiologists in fermentation cultures of Streptomyces species (Hata et al., 1956). It also is known as 7-amino-9α-methoxymitosane (Majmudar et al., 2015). This drug has been used as a chemotherapeutic agent (Verweij and Pinedo, 1990), due to its alkylating and deoxyribonucleic acid (DNA) cross-linking antiproliferative activities first discovered by Iyer and Szybalski (1963).
Figure 1.
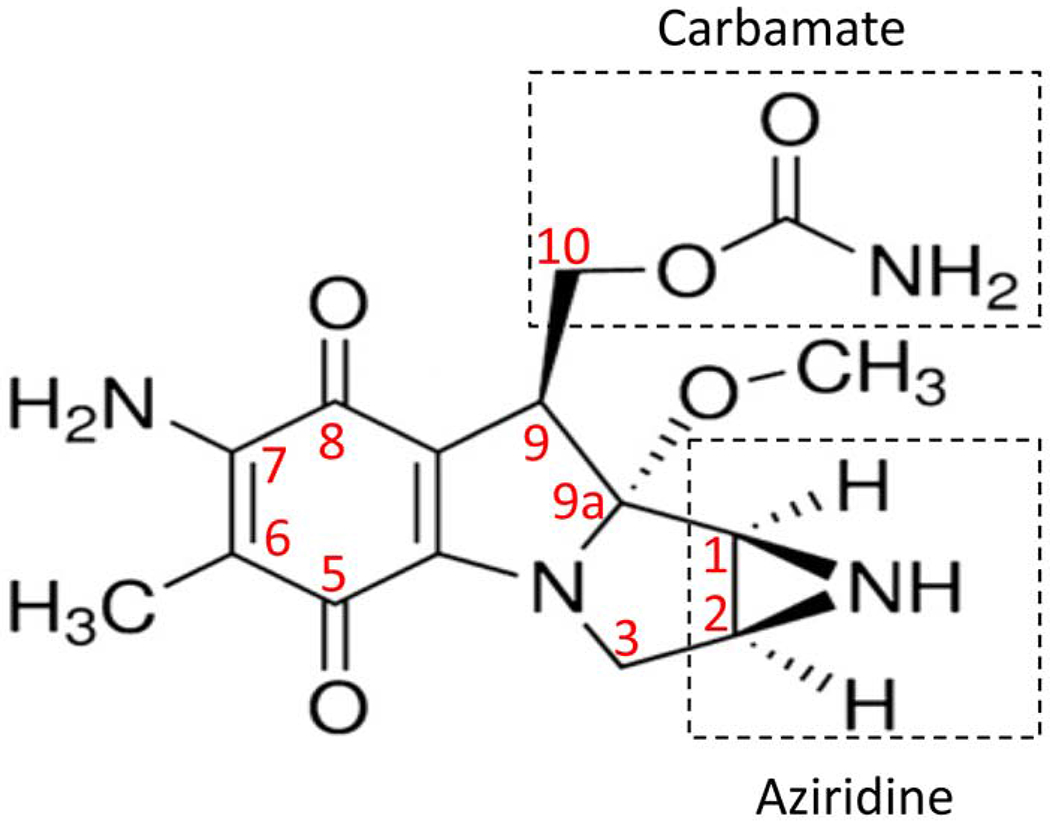
The chemical structure of mitomycin C as reported by (Galm et al. (2005) and Tomasz and Palom (1997).
An alkylating agent attaches an alkyl group (CnH2n+1) to the guanine nucleotide of DNA, which can result in a covalent cross-link between two guanine nucleotides in DNA strands (Galm et al., 2005; Tomasz and Palom, 1997). In the process of normal DNA replication during cellular division, the DNA double-helix is separated into single strands by enzymes that break the hydrogen bonds between the nucleotides of complementary DNA strands. The presence of an inter-strand covalent bond, such as that produced by an alkylating agent, prevents the DNA double-helix from uncoiling and separating. Since this is required for DNA and cellular replication, the cell with cross-linked DNA can no longer proliferate (Galm et al., 2005; Tomasz and Palom, 1997; Galm et al., 2005; Iyer and Szybalski, 1963; Tomasz and Palom, 1997).
There are two types of alkylating agents: mono- and bi-alkylating. The former can react only with one guanine, whereas the latter can react with two different N-guanine residues. If this occurs in the two different strands of the DNA, the result is cross-linkage of the DNA strands. However, the bi-alkylation and guanine-drug-guanine binding can be formed in the same DNA strand, which is called “limpet attachment”. Only bi-alkylating agents can cause cross-linking of DNA strands, but mono-alkylation and limpet attachment can also prevent vital DNA-processing enzymes from accessing the DNA, and, thus, also inhibit cellular replication or lead to cell death (Basu et al., 1993).
The specific mechanisms of MMC-induced DNA damage have been thoroughly investigated (Seow et al., 2004; Tomasz and Palom, 1997). MMC-induced DNA damage occurs through both mono- and bi-functional alkylations (Seow et al., 2004; Tomasz and Palom, 1997). Iyer and Szybalski (1964) first hypothesized that mono-alkylations initially occur through the C-1 aziridine group of MMC (Fig. 1) to the N2 position of guanine base in DNA, and may proceed to bi-alkylation to form a DNA cross-link through the C-10 of carbamate group of MMC (Fig. 1) to an adjacent DNA guanine at its N2 position (Iyer and Szybalski, 1964; Seow et al., 2004; Tomasz and Palom, 1997). Molecular modeling studies have indicated that these bindings occur in the minor groove, and cause remarkably little perturbation in the structure of the duplex DNA (Tomasz and Palom, 1997).
Mitomycin C itself does not react with DNA. It is a pro-drug and requires enzymatic or chemical reduction of its quinone ring to be transformed into a highly-reactive alkylator with DNA cross-linking activities (Iyer and Szybalski, 1964; Seow et al., 2004; Tomasz and Palom, 1997). Numerous tissue enzymes have been shown to be capable of reducing MMC, including NADPH:cytochrome P450 oxidoreductase, NADPH:cytochrome b5 oxidoreductase, DT-diaphorase, xanthine:oxygen oxidoreductase, xanthine:NAD+ oxidoreductase, nitric oxide synthase, and NADPH-ferrodoxin (Ross et al., 1996; Seow et al., 2004). A hypoxic state was reported to favor the reductive processes (Kennedy et al., 1980b, 1980a), and, therefore, an unique feature of MMC is its conversion to the active drug through an enzymatic reduction process that preferentially proceeds in the absence of oxygen.
MMC-induced DNA cross-links are also believed to possibly induce programmed cell death (apoptosis) in rapidly proliferating cells (Seow et al., 2004). A single cross-link per genome has been reported to be sufficient to cause bacterial cell death (Seow et al., 2004; Szybalski and Iyer, 1964). Excellent correlation has been reported between DNA inter-strand cross-linking activity and the level of mammalian cellular death (Seow et al., 2004; Tomasz and Palom, 1997). A single, site-specific MMC cross-link that was inserted synthetically was found to be 60-fold more lethal than the alkylation by a corresponding monofunctional agent (Tomasz and Palom, 1997). As a result, bi-alkylation and DNA inter-strand cross-linking has been considered more cytotoxic than mono-alkylation (Tomasz and Palom, 1997). Although DNA alkylation can occur at any stage of the cell cycle, the biological consequences have been found to be most severe during DNA synthesis, particularly at the late G1 and S phases (Mladenov et al., 2007). Regardless of the phase of the cell cycle during which the DNA inter-strand crosslinks are induced, cells are likely to be arrested in the S-phase of mitosis (Mladenov et al., 2007).
MMC-induced apoptosis is thought to be mostly related to the mitochondrial pathway mediated by caspases (Kim et al., 2003; Woo et al., 2014). Additional mechanisms for MMC cytotoxicity have also been proposed, such as their ability to generate oxygen radicals or amplify tumor necrosis factor (TNF) production (Pogrebniak et al., 1991; Pritsos and Sartorelli, 1986). MMC treatment is unlikely to impair DNA transformation and protein synthesis because MMC-induced alkylation appears to interfere with only a small part of the entire genome DNA, and these cited activities reflect a function of a relatively small fraction of the total DNA complement (more than 98% of human DNA is non-coding). Corroborating this statement, only little effect on the ability of DNA to transform or direct the synthesis of proteins were seen in bacterial colonies treated with MMC (Iyer and Szybalski, 1963). However, under hypoxic conditions and/or at high drug concentrations, cellular RNA and protein synthesis may also be suppressed (Netto et al., 2006b).
While MMC effects are beneficial against undesirable pathogens or tumor proliferative cells, they may also be toxic to normal cells, particularly cells that divide frequently, such as those in the embryo or in adult gastrointestinal tract, bone marrow, testicles and ovaries. When applied to the cornea, there is a premise that MMC could trigger apoptosis of practically all cells which it enters in high concentrations, including keratocytes, endothelial cells, corneal epithelial cells, limbal steam cells and conjunctival goblet cells.
Since MMC is nonreactive in its natural oxidized state, the bio-reductive metabolism of the drug is the first step in expression of cytotoxicity (Ross et al., 1996). Research should be devoted to this subject, with a focus on specific enzymes involved in activation of the drug in the cornea. Knowledge of the role of a particular reductase, and whether such a reductase is abundant in a particular cell type, for example corneal fibroblasts or myofibroblasts, could be used for developing enzyme-directed anti-fibrotic therapy with MMC.
3. Corneal wound healing following PRK
Corneal surface ablation surgeries incite immediate and long-term changes in the cornea at the molecular and cellular level. The corneal wound healing response, including that associated with PRK, has been covered in detail elsewhere in these special issues (Wilson, 2020a,b), and only relevant details will be mentioned here.
PRK surgeries begin with mechanical removal or laser ablation of the epithelium and subsequent laser ablation of any remaining EBM and Bowman’s layer (if the species has one), along with a variable portion of the anterior stroma—depending on the type and degree of attempted correction. Subsequently, a cascade of events takes place that includes the death of keratocytes, immune cell infiltration, activation of stromal cells surrounding of the ablation zone, and at least early stages of myofibroblast generation. The epithelium quickly heals over the wound and EBM regeneration, co-coordinated by the epithelium and underlying corneal fibroblasts/keratocytes, begins. Immediately after PRK, the anterior keratocyte population underlying the excimer laser ablation undergoes apoptosis (Wilson et al., 1996) and, importantly, the level of keratocyte death is dependent on the amount of excimer laser stromal ablation (Mohan et al., 2003). Whether or not keratocytes fully-repopulate the anterior stroma to preoperative levels remains controversial (Amoozadeh et al., 2009; de Benito-Llopis et al., 2012; Erie, 2003; Erie et al., 2003; Midena et al., 2007). However, complete restoration of mature epithelial basement membrane, corneal nerves, and stromal remodeling takes months to years depending on the magnitude of the stromal healing response. Early clinically insignificant haze, attributable to the increased opacity of corneal fibroblasts themselves and the limited disorganized extracellular matrix they produce (Jester et al., 1999), occurs in all corneas within the first few months after PRK, and typically resolves over 6 to 12 months. The clinical use of MMC on the ablated stroma has been shown to remarkably diminished the incidence of late haze stromal fibrosis after PRK procedures (Carones et al., 2002).
In the moments following PRK ablation, keratocyte apoptosis is detected surrounding the stromal ablation (Wilson et al., 1996; Helena et al., 1998; Kim et al., 2003). Epithelium that is injured (either by mechanical scrape, laser injury or alcohol removal) releases interleukin (IL)-1α, IL-1β, and possibly other modulators, that triggers subjacent keratocytes to undergo apoptosis mediated by the Fas-Fas ligand system (Wilson et al., 1996; Wilson et al., 2003). Some investigators have suggested that ultraviolet laser radiation, such as an excimer laser beam, also triggers keratocyte apoptosis via oxygen free radicals produced by the ultraviolet radiation itself and possibly by thermal increases, especially with early models of excimer lasers (Bilgihan et al., 2002, 2003, 1996; Hayashi et al., 1997).
Mohan et al. (2003) studied keratocyte apoptosis, keratocyte mitosis and other parameters in rabbit corneas at 4, 24 and 72 hours, 1 and 4 weeks, and 3 months following PRK and LASIK. Importantly, PRK results in higher levels of keratocyte apoptosis, stromal cell proliferation and bone marrow-derived cell stromal influx than LASIK, and the level of each of these responses increased with the level of attempted correction for myopia. In recent studies it was observed that bone marrow-derived CD11b-positive cells (including monocytes, macrophages, granulocytes, lymphocytes, and fibrocytes) enter the stroma in large numbers in rabbits or mice after either −4.5D, −9D PRK or irregular phototherapeutic keratectomy (PTK) (Lassance et al., 2018; de Oliveira and Wilson, 2020; de Oliveira and Wilson, unpublished data, 2020). This influx of bone marrow-derived cells peaks within 2 to 3 days after PRK, but continues a low levels for at least a week after surgery. Chemokines produced by surviving keratocytes and progeny corneal fibroblasts upon IL-1 or TNF alpha stimulation (such as monocyte chemotactic and activating factor or granulocyte colony stimulating factor) attract the bone marrow-derived cells into the cornea, primarily from the limbal blood vessels (Hong et al., 2001; Wilson et al., 2001; Hayashi et al., 2010; Mack, 2018). The level of “inflammation” or bone marrow-derived cell influx is known to be related to fibrosis in different organs (Mack, 2018).
Importantly, among the countless bone marrow-derived cells that enter the stroma, fibrocytes, along with corneal fibroblasts derived from keratocytes, are the progenitors to myofibroblasts that produce the disordered extracellular matrix associated with fibrotic late haze (Lassance et al., 2018; Wilson, 2012; Wilson et al., 2001). Importantly, these progenitor cells must also proliferate to give rise to sufficient myofibroblasts to trigger a clinically significant fibrosis response (Mohan et al., 2003, Lassance et al., 2018; Wilson, 2020c, Andresen et al., 1997; Kim et al., 1999; Marino et al., 2017, 2016; Medeiros et al., 2018b; West-Mays and Dwivedi, 2006; Wilson, 2019).
Soon after stromal injury, quiescent keratocytes in proximity to the excimer laser ablation proliferate and become “activated” to corneal fibroblasts—likely driven by epithelium-derived growth factors such as transforming growth factor beta (TGFβ)-1 and -2, basic fibroblast growth factor (FGF-2), platelet derived growth factor (PDGF), and insulin-like growth factor 1 (IGF-1) (West-Mays and Dwivedi, 2006; Jester and Ho-Chang, 2003; Stern et al., 1995). These corneal fibroblasts, along with fibrocytes, proliferate and begin a developmental program to become mature, extracellular matrix secreting myofibroblasts that produce opaque stromal fibrosis (Carlson et al., 2003; Fini, 1999; Jester et al., 1999; West-Mays and Dwivedi, 2006). These developing myofibroblast progenitors are dependent on an ongoing source of TGFβ1 and/or TGFβ2 to continue their development, and for their persistence once they are fully-developed, or they undergo apoptosis (Jester and Ho-Chang, 2003; Wilson, 2020c).
Whether the EBM is regenerated in a timely manner, cutting off the requisite supply of tear and epithelial TGFβ1 and TGFβ2, is a major determinate of whether sufficient mature myofibroblasts develop to generate clinically significant stromal fibrosis (Torricelli et al., 2013; Marino et al., 2016,2017). Defective EBM regeneration is attributable to inadequate production or incorrect assembly of BM components, including laminins, nidogens, perlecan and collagen type IV (Torricelli et al., 2916; Marino et al., 2016). Defective EBM regeneration correlates with the severity of stromal injury, stromal surface irregularities, as well as delayed re-epithelization (Netto et al., 2006a; Wilson, 2018). Thus, after low correction PRK with smooth ablations, and if the epithelium heals promptly, the EBM and epithelial barrier function (EBF) are quickly regenerated. Consequently, the ongoing supply of TGFβ1 and TGFβ2 from the tear and epithelium is interrupted, corneal fibroblasts and fibrocytes undergo apoptosis (or possibly revert to their respective progenitor cells), and the wound healing response subsides without the development of myofibroblasts or their production of large amounts of disordered extracellular matrix (Marino et al., 2017, 2016; Medeiros et al., 2018b; Wilson, 2019). Conversely, following high corrections for myopia with greater stromal cell loss, irregular ablations (including high corrections of astigmatism), or delayed epithelial healing, the EBM and EBF are not restored in a timely manner, resulting in sustained delivery of tear and epithelial TGFβ1 and TGFβ2 to the anterior stroma—which drives mature myofibroblast development and maintains their viability (Marino et al., 2017, 2016; Medeiros et al., 2018b; Wilson, 2019). In rabbit corneas, this process is typically detectible at two weeks after surgery and peaks at approximately 4 weeks after the procedure (Marino et al., 2017, 2016; Medeiros et al., 2018b; Wilson, 2019). Fibrotic stromal haze typically develops later in human corneas, with the maximal response usually being from two to four months after surgery (Marino et al., 2017, 2016; Medeiros et al., 2018b; Wilson, 2019).
Eventually, the EBM and EBF may be fully-restored as normal keratocytes make their way through the fibrosis to cooperate with the epithelium in regenerating the EBM, resulting in decreased stromal TGFβ levels, and the resulting apoptosis of mature myofibroblasts. Keratocytes then reabsorb the fibrotic extracellular matrix and restore corneal transparency in a process that can take years (Hassell and Birk, 2010). In some corneas, fibrosis persists indefinitely and the cornea remains permanently opaque.
4. Mitomycin C inhibition of corneal fibrosis after PRK
MMC’s primary effect after PRK is to inhibit the proliferation of myofibroblast progenitor cells, and thereby inhibit mature myofibroblasts generation (Netto et al., 2006b). Some investigators found a small, but statistically significant, increase in the early keratocyte apoptosis response in the anterior stroma of MMC-treated corneas compared to controls (Chang, 2005; Kim et al., 2003; Netto et al., 2006b), others found no difference in the number of apoptotic cells or early keratocyte loss between MMC-treated and control groups (Blanco-Mezquita et al., 2014; Rajan et al., 2006). Thus, the profound decrease in Ki67-positive mitotic stromal cells and a subsequent major decrease in myofibroblasts (Fig 2) appears to be the most important MMC effect (Netto et al., 2006b). The use of 0.02% MMC eye drops after complete re-epithelization was found not to inhibit fibrosis generation (Kim et al., 2004), probably because stromal cellular proliferation peaks at 24 to 72 hours after PRK (Mohan et al., 2003) and/or because the MMC did not penetrate well into the stroma. Blanco-Mezquita et al. (2014) also found evidence of MMC reducing the initial stromal cellular proliferation response. In addition, a confocal microscopy study found less conspicuous “activated keratocytes” and ECM in MMC-treated corneas compared to untreated controls (Gambato et al., 2005).
Figure 2. Histopathological and clinical findings following 0.02% MMC and vehicle control applications in rabbit corneas that had −9D PRK.
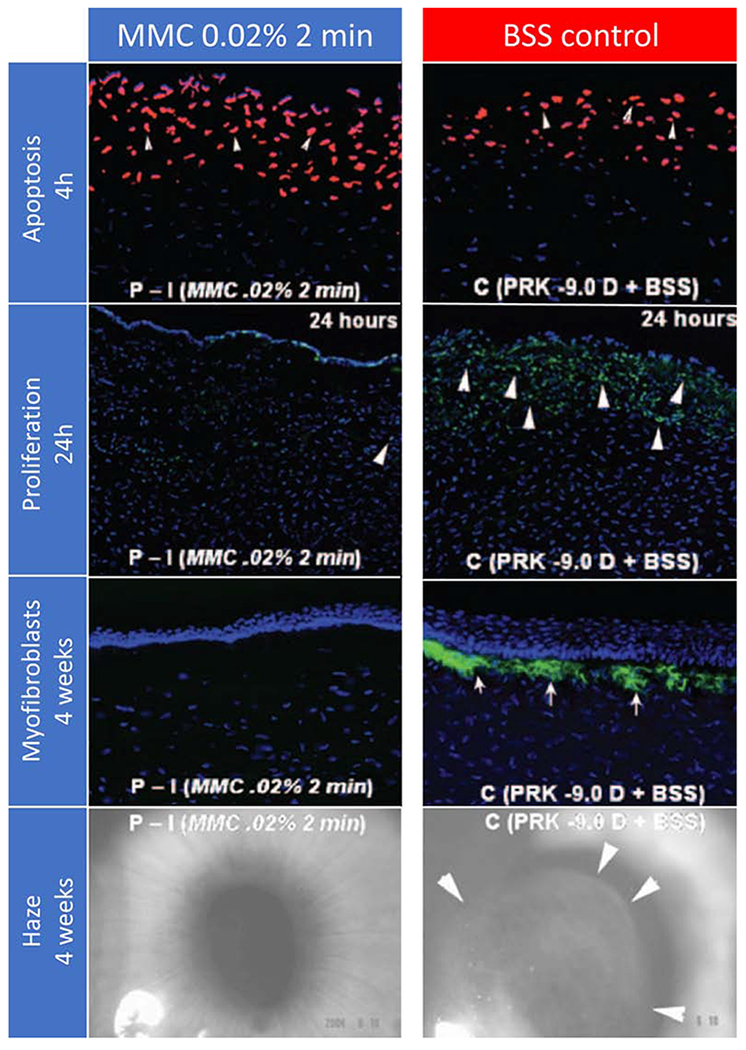
First row. Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay to detect fragmented DNA characteristic of apoptotic cells four hours after −9D PRK and 0.02% mitomycin C for 2 minutes or −9D PRK and vehicle balanced salt solution (BSS) application for 2 minutes in rabbits. Cell nuclei were stained blue with DAPI and TUNEL-positive cells stained red (arrows). There was a small but statistically significant increase in the number of cells undergoing apoptosis in the stroma in the mitomycin C group compared to the control group, with the cornea with the greatest level of apoptosis shown (original magnification 200 X). Second row. Immunohistochemistry for Ki-67 at 24 hours to detect cell mitosis in the central cornea of rabbit eyes that had −9 D PRK for myopia followed by mitomycin C 0.02% or vehicle application for 2 minutes after treatment. There was heavy cell mitosis (arrowheads) in the anterior stroma in the vehicle-treated group (C). Markedly reduced proliferation of cells in the anterior stroma was noted in corneas treated with 0.02% mitomycin C for 2 minutes (P-I). Third row. Immunohistochemical staining for the alpha-smooth muscle actin (α-SMA) marker for myofibroblasts in rabbit corneas that were treated with MMC 0.02% or vehicle BSS for 2 minutes after −9D PRK. Cells nuclei were stained blue with DAPI and α-SMA-positive myofibroblasts were stained green (arrows). Note that no myofibroblasts were seen in the MMC-treated corneas, whereas numerous α-SMA+ myofibroblasts were detected in all corneas in the BSS-treated group (original magnification 200X). Fourth row. Representative slit-lamp photographs show subepithelial haze patterns and locations restricted to the area of corneal ablation. Faint 0.5 subepithelial corneal haze is present in the prophylactic mitomycin C −9D PRK group P-I while Grade III subepithelial haze was present in all corneas in the prophylactic BSS-treated PRK group (C). Note that the subepithelial haze tends to terminate exactly at the margin of the excimer laser ablation (arrows in C) (original magnification 10X). Reprinted with permission from Netto et al. J. Refract. Surg. 2006;22:562–574.
5. MMC dosage and exposure time in fibrosis prophylaxis
5.1. Clinical efficacy
Multiple studies have shown that the immediate post-ablation application of MMC to the stroma significantly reduces haze formation in human eyes undergoing PRK surgery for high myopia, hyperopia and high astigmatism treatments (Bedei et al., 2006; Carones et al., 2002; Chen et al., 2011; Gambato et al., 2005; Hashemi et al., 2017, 2004; Kaiserman et al., 2017; Leccisotti, 2009; Lee et al., 2005; Majmudar et al., 2000, 2015; Sorkin et al., 2019). Nevertheless, the optimal concentration and exposure time for prophylactic MMC treatment continue to be topics of discussion.
Several studies evaluated the effects of different MMC concentrations and stromal exposure times on the development of fibrosis. Netto et al. (2006b) studied the effects of prophylactic MMC on stromal cellular proliferation, subepithelial stromal opacity and myofibroblasts generation after −9D (108μm ablation depth) PRK in rabbits. The study compared 0.02% and 0.002% MMC, applied for 12 seconds, 1 or 2 minutes. The shorter exposure time of 12 seconds was as effective as the longer exposure time of 2 minutes in reducing subepithelial opacity documented at the slit lamp in rabbits. IHC measurement of Ki-67 mitotic cells and alpha-smooth muscle actin (SMA)-positive myofibroblasts showed that MMC application for 12 seconds showed a trend towards more Ki-67-positive proliferating cells at 24 hours, as well as a higher numbers of myofibroblasts in the anterior stroma at 1 month compared to the 1 or 2 minute MMC applications. But since the difference was not statistically significant, shorter exposure times were hypothesized to be just as effective as the longer times in preventing fibrosis (Netto et al., 2006b). Virasch et al. (2010) also evaluated the relationship between fibrosis formation and MMC contact time. This retrospective study contained 269 eyes that had PRK with standard dose 0.02% MMC applied for 12 seconds, 1 minute or 2 minutes for eyes with a mean spherical equivalent of −6.5 to −7.1 diopters of myopia. The authors found no difference in average subjective categorical opacity scores or best-corrected visual acuity (BCVA) between the groups. However, considering the relatively low incidence of fibrosis, the relatively small sample size was a limitation of the study. A double-masked, randomized prospective trial evaluated the efficacy of 0.01% MMC applied for 60-, 30- or 15 seconds in preventing fibrosis formation after PRK for moderate to high myopia (SE range −4.40 to −8.00 D) (Hofmeister et al., 2013). Only 28 patients were enrolled, and no difference in opacity was found between the three MMC exposure times, but the relatively low patient numbers could have hindered the investigators from finding a difference.
Kaiserman et al. (2017) reported a retrospective study containing 7535 eyes that had PRK with MMC. Among other observations, the authors evaluated the incidence and severity of corneal haze relative to MMC intraoperative application time (above or below 40 seconds). The surgeries were performed with 0.02% MMC placed in the center of the cornea and applied for 20 to 60 seconds, according to the amount of ablation and surgeon’s preference. The reported incidence of haze in the groups with MMC applied for shorter or longer periods were: 2.7% (25/941) versus 1.4% (10/707) in eyes with high myopia (RR 1.92, P <0.05); 1.3% (34/2560) versus 0% in those with moderate myopia (P = 0.03); 1% (25/2589) versus 2.3% (5/217) (RR 0.44) in those with low myopia; and 12.3% (9/73) versus 0% (0/97) in those with hyperopia. Significant differences in efficacy and in safety between the severe late haze and the no-haze groups were noted. Thus, from this large cohort of patients, the authors concluded that among other factors, short MMC application times were evidently associated with an increased incidence of “breakthrough haze”, where significant late haze develops despite MMC treatment, and longer MMC application times might have been beneficial for haze prevention in eyes with higher corrections.
Although high myopia has been linked to higher risk of haze development, Shojaei et al. (2013) studied the relevance of very short application of 0.02% MMC for preventing haze in patients with low myopia (ablation depth <65 μm). They conducted a prospective, randomized, sham-controlled, double-masked clinical trial to assess the benefit of 0.02% MMC applied for 5 seconds in preventing haze for low-risk eyes group (Shojaei et al., 2013). One hundred eighty-four patients were randomized to receive a 5-second MMC application versus balanced salt solution, with 152 patients completing the 6-months follow-up (78 and 74, respectively). There was no statistically significant difference in CDVA between the groups, but haze grade was significantly lower in the MMC treated group at 3 and 6 months after surgery. Only 1 eye (1.4%) had trace haze in the MMC treated group versus 9 eyes (11.8%) in the control group at the final 6-month follow-up. The authors suggested that cornea haze after PRK for low myopia, although usually clinically insignificant, should not be overlooked, and that a short application of 0.02% MMC was safe and effective in preventing haze formation in eyes having PRK with an ablation depth of less than 65 μm.
Several authors have also evaluated the effects of changing the concentration of prophylactic MMC on the resultant corneal haze formation. Netto et al. (2006b) found no significant difference in haze development by using MMC at 0.02% or 0.002% after −9D PRK performed in rabbit corneas. Although the lower concentration of 0.002% was as effective as 0.02% MMC when used for the same exposure times, opacity was statistically lower when 0.02% MMC was used for 2 minutes than 0.002% MMC used for 12 seconds. The authors suggested that with larger size samples in each prophylactic group, statistically significant differences in subepithelial haze might have been detected between the different MMC’s concentrations or exposure times, but these differences would most likely not have been clinically relevant (Netto et al., 2006b).
Thornton et al. (2008) retrospectively compared the efficacy of low-dose 0.002% MMC (126 eyes) to the standard-dose 0.02% MMC (95 eyes). In both groups, MMC was applied to the stromal bed for either 30 seconds or 2 minutes, at the discretion of the surgeon. Postoperatively, less haze was noted in the standard 0.02% MMC group for eyes with high myopia (≥ −6.00 D) and higher ablation depths (≥ 75 μm). Although eyes with moderate myopia (−3.00 D to −5.90 D) had a lower median haze score at 1 and 3 months when treated with the standard 0.02% MMC dose, no statistically significant differences were noted in the later postoperative follow-ups. Apart from haze, there was no statistically significant difference in efficacy ratio (defined as postoperative UCVA divided by the preoperative BSCVA) between the low- and standard-dose eyes at all postoperative time points for the total cohort and among the subgroups. The authors also prospectively compared low-dose 0.002% MMC for 30 seconds in one eye with same dose (0.002% MMC) for 2 minutes in the other eye (Thornton et al., 2008). This evaluation found no significant difference in haze between these different exposure times, but safety ratio (postoperative BSCVA/preoperative BSCVA) at 3-month follow-up was statistically better in the 2 minutes group. The authors concluded that the standard concentration of topical 0.02% MMC was more effective than low dose 0.002% MMC in preventing postoperative haze for myopia ≥−6.00 D and ablation depths ≥75 μm. They recommended that low dose concentration only be used in selected cases of low to moderate myopia and shallow ablation depth, since it was often not sufficiently potent to retard the formation of haze in other situations. This, however, was a relatively small study, especially given the extremely low incidence of postoperative haze after low to moderate PRK corrections for myopia, which could have impacted the results. In a prospective study, Coelho and Sieiro (2019) evaluated the efficacy of 0.002% MMC compared to 0.02% MMC applied for 30 seconds in preventing late haze fibrosis after PRK and found no significant difference between the groups. Similarly, Razmjoo et al. (2013), in a contralateral eye study, examined 0.02% MMC compared to 0.01% MMC applied for 45 seconds in reducing opacity after PRK in 170 eyes of 85 patients. Grade 1 clinical opacity was seen in 14 eyes (16.5%) in the 0.01% MMC group compared to 7 eyes (8.2%) in the 0.02% MMC group at 3 months, but no visual performance differences were noted between the two groups.
Several points are worth noting when evaluating these studies. (1) The low incidence of haze in human eyes makes it difficult to compare the efficacy of different MMC concentrations or exposure times in reducing opacity. For example, Kaiserman et al. (2017) in a large cohort study reported a haze incidence of 2.1% of eyes treated for high myopia and 1.1% in eyes treated for low to moderate myopia. In that study, the incidence of level 2 or 3 opacity was even lower—1.03%, 0.14%, and 0.10% in eyes with high, moderate or low myopia, respectively (Kaiserman et al., 2017). Thus, large numbers of corneas would be needed to detect clinically significant differences between different MMC concentrations or exposure times; (2) Unmasked evaluation of the postoperative haze by slit lamp examination is subject to potential observer bias. Therefore, prospective, randomized, double-masked studies and objective haze quantification by more objective methods such as optical coherence tomography (OCT) would be of interest to study the role of different MMC protocols; (3) The efficacy of MMC as a haze-reducing agent is also influenced by factors other than MMC concentration and exposure time. These include the environment where the patient resides after PRK (higher exposure to ultraviolet radiation may yield a higher late haze risk), variations in epithelium removal technique could be important (Kaiserman et al., 2017), characteristics of the device used to apply MMC (Khoury et al., 2007), and the importance of ablation zone diameters and laser platforms in the development of late haze fibrosis (Hofmeister et al., 2013). These other factors should be considered in comparing different studies of MMC concentration and exposure times.
MMC use in PRK for low myopia remains controversial, with many clinicians continuing to feel it is unnecessary for eyes with less than 5D to 6D of myopia. The incidence of opacity after surface ablation for hyperopia was shown to be higher than for surface ablation for myopia (Kaiserman et al., 2017). Opacity after PRK for hyperopia is typically located in the mid-peripheral cornea, but it can cause significant refractive regression. More studies with large numbers of corneas are needed to more precisely determine the optimal MMC concentrations and exposure times in PRK retreatments.
5.2. MMC safety
Mitomycin C has been used for PRK in human cornea for almost 20 years (Carones et al., 2002; Majmudar et al., 2000) without serious complications being reported (Gambato et al., 2011; Hashemi et al., 2017, 2016; Lee et al., 2005; Sorkin et al., 2019). A remaining question is whether MMC’s effects occur only in rapidly proliferative cells or whether there are persisting effects on resting cells that could result in long-term effects from DNA damage in surviving cells. This question is clinically relevant since the MMC-induced DNA damage could augment cumulative ultraviolet-induced DNA injury in epithelial cells, keratocytes or endothelial cells that could produce adverse effects decades later—such as corneal thinning due to diminished keratocyte density or corneal edema due to endothelial cell loss.
5.2.1. MMC effects on the corneal epithelium
Epithelial cells have a rapid turnover rate, which could make this tissue particularly vulnerable to MMC treatment. Woo et al. (2014) in vitro studies showed that cultured human corneal epithelial cells treated with MMC undergo apoptosis in a concentration- and time-dependent fashion. Rajan et al. (2006), using an in vitro human cornea healing model, documented potential effects of 0.02% MMC applied for 1 or 2 minutes during PRK on epithelial healing. MMC-treated corneas had significantly delayed epithelial latency (the time until the start of epithelial migration) and slower migration, with a consequent delay in wound closure compared to the untreated control group.
Conflicting results have been reported in animal studies. Chang (2004) evaluated possible adverse effects of 0.01% or 0.02% MMC applied for 2 minutes compared to balanced salt solution for 2 minutes in pigmented rabbit corneas, and found a dose-dependent delay in corneal re-epithelialization. Conversely, Blanco-Mezquita et al. (2014) did not find any delay in epithelial healing in hen eyes treated with 0.02% MMC for 12 seconds.
A large clinical retrospective study compared epithelial healing in 1520 eyes that had PRK for myopia with 0.02% MMC for 20 seconds to 500 myopic eyes that had PRK without MMC (Kremer et al., 2012). The healing time for epithelial defect ranged between 5 and 7 days in both groups, except for a few cases with delayed closure (up to 14 days). When the incidence of these cases was compared between the two groups, 0.02% MMC applied for 20 seconds was found to associated with delayed epithelial wound healing in a higher number of eyes. However, no increase in stromal opacity was found in the MMC-treated eyes. Lee et al. (2005) reported only two cases (0.2%) of delayed epithelial healing in a large case series that included 1011 eyes which had PRK with intraoperative application of 0.02% MMC for 30 seconds to 2 minutes. Also, small clinical studies found no difference in re-epithelization when MMC was applied after PRK. Thus, Carones et al. (2002) reported a prospective, randomized, masked study containing 60 consecutive eyes with spherical equivalents between −6D and −10D. The study compared eyes that had PRK with 0.02% MMC for two minutes to eyes that had PRK without MMC treatment. They found complete re-epithelialization in all eyes by the fifth day, with no differences in the quality of re-epithelialization, pain, or toxicity. Gambato et al. (2005) also investigated the effects of 0.02% MMC for 2 minutes compared to placebo on epithelial healing. This prospective, double-masked, randomized clinical trial included 72 eyes. Corneal re-epithelialization was completed within 5 days in both groups, and there was no difference between the two groups in the quality of re-epithelialization, pain, or toxicity during re-epithelialization. Finally, Leccisotti (2008) conducted a prospective, randomized, double-masked, paired eye study to evaluate the effect of topical 0.02% MMC for 45 seconds after PRK on epithelialization in fifty-two eyes with high myopia. The mean epithelialization time was identical in the MMC and placebo groups (3 days, with a range of 2–4 days).
Another consideration when evaluating potential adverse effects of MMC on the epithelium is its potential effect on epithelial morphology. Epithelial layer changes have been described following PRK, and are correlated with smaller ablation zones, lack of blending at the edge of the ablated zone, and higher attempted corrections associated with deeper stroma ablations (Dawson et al., 2005; Gauthier et al., 1997; Gipson, 1990; Lohmann et al., 1999). The epithelium can become thicker following PRK due to basal epithelial cell hypertrophy and epithelial hyperplasia (Dawson et al., 2005). Since the epithelium contributes to the total corneal dioptric power (Gatinel et al., 2007; Legeais et al., 1997), epithelial thickening may produce at least transient postoperative refractive under-correction after PRK and other surface ablation procedures for myopia (Lohmann et al., 1999). Potential MMC effects in preventing post-operative epithelial thickening have not been well studied. Rajan et al. (2006), using a human in vitro model, found the epithelial layer was poorly differentiated and significantly thinner in the eyes that received 0.02% MMC for 2 minutes compared to untreated control eyes. More studies are needed to address this possible early MMC effect. However, with 5 years of follow up, epithelial thickness was found to be unchanged in corneas treated with PRK and MMC (Gambato et al., 2011).
Thus, there appears to be no compelling evidence that there is significant epithelial toxicity from the use of MMC in PRK that is comparable to the benefits noted in preventing PRK-induced stromal fibrosis. It is, however, prudent to use a sponge to apply the medication to prevent contact with the peripheral corneal epithelium and limbus (Khoury et al., 2007).
5.2.2. MMC effects on goblet cells, corneal nerves and the tear film
The possibility that MMC could be toxic to goblet cells motivated Mohammadi and colleagues (2014) to investigate the effects of MMC on the tear film. In a double-masked, randomized clinical trial, sixty patients with low to moderate myopia had PRK with 0.02% MMC or vehicle for 15 seconds. Patients were evaluated with a Schirmer’s test, tear breakup time test and a symptoms questionnaire at baseline, one- and six-months postoperatively. The authors reported comparable tear-film indices in the MMC and control groups at both postoperative examinations, and all tear-film indices returned to the baseline values by six months in both groups.
Farahi et al. (2013) carried out a prospective, randomized, double-blinded study to assess the effects of adjuvant MMC on tear function after PRK for low to moderate myopia. Twenty-seven patients underwent PRK in one eye and PRK plus 0.02% MMC for 20 seconds in the contralateral eye. Eyes were assessed with corneal esthesiometry, fluorescein break-up time, fluorescein and rose bengal staining corneal staining, as well as the Schirmer’s test with anesthesia preoperatively and at 1.5, 3, 6, and 12 months after PRK. Patients also submitted a questionnaire at each follow-up. When preoperative and postoperative results were compared, statistically significant differences were only observed in fluorescein break-up time and dry eye symptom score at 1.5 months, and both of these indices showed improvement at the later time points. Corneal sensation was significantly lower at all postoperative follow-up visits compared with preoperative values. However, when symptoms and tear function tests were compared between groups, no statistically significant differences between the groups were noted at any visit.
During surface ablation procedures, epithelial removal and PRK ablation removes all nerves in the epithelium and sub-basal layer of the central cornea, as well as deeper stromal nerve fibers depending on the ablation depth. The recovery to preoperative densities and morphology typically takes 6 to 12 months (Erie et al., 2005; Gambato et al., 2011; Medeiros et al., 2018a). It has been proposed that MMC could actually facilitate nerve regeneration after PRK since myofibroblasts have been shown to interfere directly with re-innervation of the epithelium (Jeon et al., 2018).
Medeiros et al. (2018a) evaluated the impact of MMC on rabbit corneal nerve regeneration after PRK. In that study, rabbit corneas that had −9D PRK with or without 0.02% MMC for 30 seconds were evaluated with acetylcholinesterase and β-III tubulin immunohistochemistry at 1-day and 1-, 2-, 3-, and 4-months after surgery (3 corneas per group at each time point). At 1-day post-PRK, the MMC-treated group exhibited significantly lower nerve density in the central ablated cornea compared to the no MMC control PRK group. However, this effect was no longer present at one month after PRK, when the corneal nerve density was found to be similar in the two groups. Therefore, although MMC had a small toxic effect on the corneal nerves when combined with PRK, the effect was only significant earlier than one month after surgery (Medeiros et al., 2018a).
Hindman et al. (2019) investigated the effect of different anti-fibrotic drugs on feline corneal nerve regeneration. The cats had PRK with intraoperative 0.02% MMC for 1 minute in 12 eyes, intraoperative prednisolone acetate (PA) followed by twice per day topical instillation for 14 days in 12 eyes, or no post-operative treatment in 14 eyes. Corneas were evaluated for late haze fibrosis (by optical coherence tomography and IHC for the myofibroblast marker a-SMA) and nerve distribution evaluated by immunohistochemistry for the nerve marker βIII-tubulin in preoperative eyes and at 2-, 4- and 12-weeks post-surgery. As expected, MMC had the greatest impact on corneal fibrosis, followed by PA, whereas untreated corneas showed greatest corneal opacity. Intraoperative application of MMC was associated with faster regeneration of corneal innervation than no treatment (Hindman et al., 2019)—likely because of MMC’s effect in reducing myofibroblasts that inhibit the nerve regeneration process.
5.2.3. MMC effects on keratocytes
Multiple in vitro studies have shown that treatment with MMC significantly increases apoptosis in cultured keratocytes in a time- and dose-dependent manner (Kim et al., 2003; Sadeghi et al., 1998; Wu et al., 1999). However, Kawase et al. (1992) showed that MMC rapidly disappeared from the ocular tissue and that tissue concentration of the chemical was significantly reduced by irrigating the tissue after treatment. Song et al. (2007) studied the concentration of MMC in rabbit corneas and aqueous humor following topical 0.02% MMC for 2 minutes. The maximal corneal MMC concentration was 3.728 ± 2.547 μg/g, which was found at 30 minutes, and decreased to only 0.756 ± 0.437 μg/g at 1 hour after application. Kampmeier et al. (2000) reported human corneal density to be 1087 kg/m3 (1.087 g/ml). Therefore, the measured MMC concentration in the cornea (3.728 μg/g) at 30 minutes after 0.02% MMC application converted to mg/ml, based on the human corneal density, would be 0.004 mg/ml (0.0004%), which is much lower than the MMC LD50 reported by Sadeghi et al. (1998). In addition, Blanco-Mezquita et al. (2014) investigated the toxicity of MMC on stromal cells in hen corneas. These investigators found no difference in the number of apoptotic cells between the groups treated with 0.02% MMC for 12 seconds and vehicle-treated controls. Thus, few keratocytes would be expected to die from MMC toxicity during PRK surgery, which has been supported by other in situ studies (Netto et al., 2006b; Song et al. 2007). Several other studies suggested that MMC application does not result in increased keratocyte loss, but that it significantly delays keratocyte repopulation in the anterior stroma (de Benito-Llopis et al., 2012; Gambato et al., 2005; Rajan et al., 2006). Thus, Gambato et al. (2005) in a prospective, double-masked, randomized clinical trial with more than 36 months of follow-up, demonstrated that topical use of 0.02% MMC for 2 minutes following PRK reduced the risk of haze formation without any added risk. Midena et al. (2007), evaluated the 5 year post-PRK effects of 0.02% MMC application for 2 minutes on corneal keratocytes in eyes with high myopia. They found that PRK surgery itself reduced keratocyte density in the anterior stroma by a small amount, but found no statistically significant difference between MMC-treated and no-MMC treated eyes.
Mohammadi et al. (2014) assessed the effects of 0.02% MMC for 15 seconds on corneal biomechanics after PRK for low to moderate myopia. No effect on biomechanical indices was found in this study.
Jester et al. (2012) studied the rabbit corneal DNA damage generation following MMC application and keratocytes ability to repair the MMC-induced DNA damage. IHC for phosphorylation of the histone (γH2AX) marker was used to assess DNA damage, while DNA repair was evaluated using the Comet assay. In cultures, keratocyte DNA damage was found to peak at 2 days following MMC use, with greater than 80% of cells exhibiting γH2AX staining. The number of cultured cells expressing γH2AX marker decreased to 40% by day 4. MMC 0.02% treatment in vivo for 15 or 60 seconds was reported to cause acute DNA damage in keratocytes in the anterior and posterior corneal stroma, as well as in endothelial cells. In contrast, MMC 0.002% did not induce DNA damage. When lamellar keratectomy was performed two months after the use of 0.02% MMC or vehicle control for 60 seconds, the corneas that were treated with MMC had decreased corneal scarring and reduced keratocyte numbers. The authors suggested this meant that the DNA inter-strand cross-links in quiescent corneal keratocytes were at least partially unrepaired at this relatively late time point. Given these findings, it’s surprising that after 20 years of widespread use of MMC during PRK no associated corneal cell malignancies have been reported. Additional long-term studies of MMC-induced DNA damage and repair are needed to better understand this inconsistency.
5.2.4. Potential MMC effects on the corneal endothelium
The potential cytotoxicity of MMC on the corneal endothelium is another concern for ophthalmologists and vision scientists that derived from in vitro or animal experiments which found MMC-related endothelial toxicity. Thus, Roh et al. (2008) reported that topical application of 0.02% MMC to intact goat globes resulted in a MMC concentration in corneal endothelial cells of 0.37 μg/ml—which was demonstrated to produce DNA cross-linking, DNA double-strand breaks and cell apoptosis. Chang (2004) reported MMC dose-dependent rabbit corneal edema and endothelial cell apoptosis. However, rabbit endothelial cells are known to have a high mitotic rate following injury, while human corneal endothelium is usually non-proliferative without pharmacologic manipulation (like after the application of Rho kinase [ROCK] inhibitors), hence, rabbit endothelial cells may be more susceptible to DNA damage induced by MMC (Chang, 2004; Santhiago et al., 2012). In an in vitro experimental study, McDermott et al. (1994) showed that human endothelial cells also responded in a dose-depend fashion to MMC administration. In that study, direct application of 200 μg/ml (0.02%) MMC on human endothelial cells resulted in prompt corneal swelling with marked ultrastructural alterations, whereas exposure to 20 μg/ml (0.002%) of MMC caused no apparent ultrastructural changes. MMC is not, however, applied directly to the endothelium in PRK. The authors suggested that although the human corneal endothelium is typically non-proliferative, the periodic repair of DNA damage—such as that induced by ultraviolet light—is still necessary, and MMC could interfere in that process.
Only two clinical studies have reported endothelial cell loss following MMC administration (Morales et al., 2006; Nassiri et al., 2008). Morales et al. (2006) performed a prospective, randomized, double-blind, placebo-controlled crossover trial to verify the risk of corneal endothelium toxicity after MMC use. Eighteen eyes were randomly assigned to receive PRK with 0.02% MMC for 30 seconds or PRK without MMC. By comparing the pre- and 1- and 3-months post-operative endothelium cell counts, the authors found a significant reduction in endothelial cells in the MMC-treated group. The authors emphasized that although the results of this study reached statistical significance, only nine subjects were enrolled, and therefore, the results needed to be confirmed in a larger prospective study (Morales et al., 2006). In a non-randomized trial that included 162 eyes with low to moderate myopia and 6-months follow-up, Nassiri et al. (2008) found that the eyes treated with 0.02% MMC for 10 to 50 seconds had more endothelial cell loss measured by specular microscopy. In that study, preoperative to postoperative changes in endothelial cell density were statistically significantly greater in the MMC-treated eyes (−14.8%) than in eyes not treated with MMC (−5.1%) at 6 months after PRK (P<0.001). Longer MMC contact time (P<0.001) and male sex (P= .04) were factors that were independently associated with greater endothelial cell loss.
In contrast, a much larger number of clinical studies have not found any evidence that endothelial damage occurred after MMC application to the bare stroma in surface ablation procedures (Ang et al., 2020; Chen et al., 2011; de Benito-Llopis et al., 2007; Diakonis et al., 2007; Gambato et al., 2011, 2005; Goldsberry et al., 2007; Hofmeister et al., 2013; Lee et al., 2005; Nassaralla et al., 2007; Shojaei et al., 2013; Zhao et al., 2008). For example, Lee et al. (2005), in a large non-comparative case series that included 1011 eyes that had PRK with 0.02% MMC for 30 seconds to 2 minutes, demonstrated that there was no significant change in endothelial cell density based on specular microscopy with a mean follow-up of 13 months (range 6 to 27 months). Of note, all eyes were evaluated at 6 months, 408 eyes had more than 6 months (mean 16 months) follow-up, and 34 eyes had 12 to 24 months follow-up. Interestingly, at 6 months, the corneal endothelial density was significantly increased compared to preoperative values, and the authors speculated this might be attributable to patients discontinuing contact lens wear after surgery. More recently, Gharaee et al. (2018), in a prospective, randomized clinical trial that included 96 eyes that had PRK for myopia with 0.02% MMC applied for 5 seconds for each diopter of SE, found that the MMC application did not affect the endothelial cell density or cell size at 6 months after surgery.
In summary, although a few in vitro and clinical studies suggested there could be endothelial cell toxicity caused by the use of MMC during PRK, the majority of the clinical studies, including those with longer follow-up (Gambato et al., 2011) and the larger size (Lee et al., 2005), found no endothelial cell loss or sight-threatening complications—such as endothelial decompensation or corneal edema—when 0.02% MMC was applied with exposure times of 2 minutes or less during surface ablation surgery.
5.2.5. MMC concentration in the aqueous humor and potential intraocular effects
MMC applied on the surface of the bare stroma has been shown to penetrate into the aqueous humor. Thus, Torres et al. (2006) used high-performance liquid chromatography to measure MMC levels in hen aqueous humor after PRK at different time points after surgery (10, 30, 60, 360, and 720 minutes). The administration of 0.02% MMC for 2 minutes after PRK resulted in an anterior chamber mean concentration of MMC of 187.250 μg/L (0.18725μg/ml) ± 4.349 (SD) at 10 minutes after the exposure. After this time point, decreasing MMC levels were detected, and MMC was un-detectable at 12 hours after the topical administration (Torres et al., 2006).
Song et al. (2007) evaluated the effects of MMC concentration and exposure time on the resulting concentration of MMC in the rabbit aqueous humor. First, the authors evaluated the concentration of MMC in the anterior chamber at different time points (0.5, 1, 2, and 3 hours) after mechanical epithelial debridement followed by the administration of 0.02% MMC on a sponge for 2 minutes. The mean aqueous MMC concentration peaked at 1 hour after the application and was 0.380 μg/mL ± 0.038. At 3 hours after MMC administration, much less MMC remained in the aqueous humor (0.017 μg/mL ± 0.010). Then, in subsequent experiments, the authors assessed the effects of different application times of 0.02% MMC (15, 30, 60 and 120 seconds) and different MMC concentrations applied for 2 minutes (0.005%, 0.01%, 0.02%, and 0.04%) on the concentration of MMC in the aqueous humor at 1 hour after the MMC application (which was the time of the peak of MMC concentration in the aqueous determined in the previous experiment). Although the concentration of MMC in the aqueous humor significantly increased with increasing exposure times and with increasing applied concentrations, a greater correlation was found between the aqueous MMC concentration and the applied MMC concentration than between the aqueous MMC concentration and the MMC exposure time. Thus, 0.04% MMC applied for 2 minutes resulted in an aqueous MMC concentration of 0.519 μg/mL ± 0.229 at 1 hour after the administration.
Kymionis et al. (2008) investigated the effects of corneal application of MMC on the ciliary body and the intraocular pressure (IOP). Forty rabbit eyes were treated with −8D PRK with application of 0.02% MMC for 2 minutes or without the application of MMC. Histological evaluation of the ciliary body was performed by light and transmission electron microscopy in a masked fashion. IOP was measured preoperatively and 5, 15, 30, and 90 days postoperatively. The authors did not find any histological or functional differences between the groups, concluding that the adjuvant application of MMC after PRK did not induce damage to the ciliary body or alterations in IOP.
Therefore, based on the available studies with adequate numbers of animals, MMC appears unlikely to produce intraocular toxicity when used during surface ablation procedures. However, data from human histopathological analyses of the iris, elements of anterior chamber angle, ciliary body, lens, vitreous humor, and retina after PRK with 0.02% MMC for exposure times up to 2 minutes are not available.
5.2.6. Potential MMC systemic effects
Crawford et al. (2013) studied the systemic absorption of MMC after topical corneal application in refractive surgery. Plasma samples were obtained from 30 patients at 30 minutes after bilateral PRK with 0.02% MMC for 30 seconds and were analyzed with liquid chromatography mass spectrometry. No MMC was detected in any patient with a sensitivity of 10.0 ng/ml using this method and, therefore, there is little likelihood of systemic toxicity when using this application method during PRK.
6. Conclusion
Mitomycin C has played a critical role in preventing stromal late haze fibrosis and limiting refractive regression after excimer laser surface ablation procedures since it was first introduced as an adjuvant to PRK treatments in 2002. The primary mode of action of MMC is to inhibit mitosis of myofibroblast progenitors and thereby decrease the generation of mature myofibroblasts that produce stromal fibrosis. The most commonly used protocol is 0.02% MMC for 30 seconds after PRK—a treatment approach found to effectively decrease scarring fibrosis in PRK, especially for eyes with more than approximately 6 diopters of myopia, without significant long-term corneal or systemic effects.
Clinical trials with large sample sizes to compare the effectiveness of different MMC protocols would be helpful to guide MMC usage by physicians. However, given the low incidence of clinically significant haze, the sample size would likely need to be very large to yield significant differences in patients. Also, additional studies are needed determine optimal MMC dosing and exposure times for PRK treatments for hyperopia and astigmatism.
MMC acts as a pro-drug and requires reduction to be converted in an active agent capable of DNA alkylation. The specific bio-reductive metabolism of the drug in corneal cells after PRK, however, has been poorly studied. Efforts to identify the specific reductase(s) that activate MMC in the cornea after topical application could lead to improvements in MMC anti-fibrotic therapy through enzyme-directed treatments, thereby increasing efficacy and limiting the drug’s toxicity.
Mitomycin C (MMC) is an alkylating agent, with DNA-crosslinking activity.
MMC inhibits DNA replication and cellular proliferation
MMC inhibits the development of mature myofibroblasts that cause stromal scarring fibrosis
MMC has been found to produce little, if any, corneal toxicity when used as adjuvant treatment in PRK
Acknowledgments
Funding
Supported in part by US Public Health Service grants RO1EY10056 (SEW) and P30-EY025585 from the National Eye Institute, National Institutes of Health, Bethesda, MD, and Research to Prevent Blindness, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Proprietary interest statement
The author doesn’t have any commercial or proprietary interests in this study.
References
- Amoozadeh J, Aliakbari S, Behesht-Nejad A-H, Seyedian M-A, Rezvan B, Hashemi H, 2009. Confocal microscopy of corneal stroma and endothelium after LASIK and PRK. J. Refract. Surg 25, S963–7. [DOI] [PubMed] [Google Scholar]
- Andresen JL, Ledet T, Ehlers N, 1997. Keratocyte migration and peptide growth factors: the effect of PDGF, bFGF, EGF, IGF-I, aFGF and TGF-beta on human keratocyte migration in a collagen gel. Curr. Eye Res. 16, 605–613. [DOI] [PubMed] [Google Scholar]
- Ang BCH, Foo RCM, Lim EWL, Tan MMH, Nah GKM, Thean LSY, Tan CWT, Zhao PSB, 2016. Risk factors for early-onset corneal haze after photorefractive keratectomy in an Asian population: Outcomes from the Singapore Armed Forces Corneal Refractive Surgery Programme 2006 to 2013. J. Cataract Refract. Surg. 42, 710–716. [DOI] [PubMed] [Google Scholar]
- Ang BCH, Yap SC, Toh ZH, Lim EWL, Tan MMH, Nah GKM, Zhao PSB, Tan MCL, 2020. Refractive outcomes, corneal haze and endothelial cell loss after myopic photorefractive keratectomy in an Asian population: The Singapore Armed Forces’ experience. Clin. Experiment. Ophthalmol [DOI] [PubMed] [Google Scholar]
- Basu AK, Hanrahan CJ, Malia SA, Kumar S, Bizanek R, Tomasz M, 1993. Effect of site-specifically located mitomycin C-DNA monoadducts on in vitro DNA synthesis by DNA polymerases. Biochemistry 32, 4708–4718. [DOI] [PubMed] [Google Scholar]
- Bedei A, Marabotti A, Giannecchini I, Ferretti C, Montagnani M, Martinucci C, Barabesi L, 2006. Photorefractive keratectomy in high myopic defects with or without intraoperative mitomycin C: 1-year results. Eur. J. Ophthalmol 16, 229–234. [DOI] [PubMed] [Google Scholar]
- Bilgihan A, Bilgihan K, Yis O, Sezer C, Akyol G, Hasanreisoglu B, 2003. Effects of topical vitamin E on corneal superoxide dismutase, glutathione peroxidase activities and polymorphonuclear leucocyte infiltration after photorefractive keratectomy. Acta Ophthalmol. Scand. 81, 177–180. [DOI] [PubMed] [Google Scholar]
- Bilgihan K, Bilgihan A, Adiguzel U, Sezer C, Yis O, Akyol G, Hasanreisoglu B, 2002. Keratocyte apoptosis and corneal antioxidant enzyme activities after refractive corneal surgery. Eye (Lond). 16, 63–68. [DOI] [PubMed] [Google Scholar]
- Bilgihan K, Bilgihan A, Akata F, Hasanreisoğlu B, Türközkan N, 1996. Excimer laser corneal surgery and free oxygen radicals. Jpn. J. Ophthalmol 40, 154–157. [PubMed] [Google Scholar]
- Blanco-Mezquita T, Espandar L, Torres R, Alvarez-Barcia A, Cantalapiedra-Rodriguez R, Martinez-Garcia C, Merayo-Lloves J, 2014. Does mitomycin C cause toxicity in the cornea after photorefractive keratectomy? A comparative wound-healing study in a refractive surgery animal model. Cornea 33, 1225–1231. [DOI] [PubMed] [Google Scholar]
- Carlson EC, Wang I-J, Liu C-Y, Brannan P, Kao CWC, Kao WWY, 2003. Altered KSPG expression by keratocytes following corneal injury. Mol. Vis 9, 615–623. [PubMed] [Google Scholar]
- Carones F, Vigo L, Scandola E, Vacchini L, 2002. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy. J. Cataract Refract. Surg. 28, 2088–2095. [DOI] [PubMed] [Google Scholar]
- Chang S-W, 2005. Corneal keratocyte apoptosis following topical intraoperative mitomycin C in rabbits. J. Refract. Surg 21, 446–453. [DOI] [PubMed] [Google Scholar]
- Chang S-W, 2004. Early corneal edema following topical application of mitomycin-C. J. Cataract Refract. Surg. 30, 1742–1750. [DOI] [PubMed] [Google Scholar]
- Chen S, Feng Y, Stojanovic A, Wang Q, 2011. Meta-analysis of clinical outcomes comparing surface ablation for correction of myopia with and without 0.02% mitomycin C. J. Refract. Surg 27, 530–541. [DOI] [PubMed] [Google Scholar]
- Coelho LM, Sieiro RO, 2019. Mitomycin C 0.02 and 0.002% efficacy in preventing haze after photorefractive keratectomy. Int. Ophthalmol 39, 341–345. [DOI] [PubMed] [Google Scholar]
- Corbett MC, O’Brart DP, Warburton FG, Marshall J, 1996. Biologic and environmental risk factors for regression after photorefractive keratectomy. Ophthalmology 103, 1381–1391. [DOI] [PubMed] [Google Scholar]
- Crawford C, Ainbinder DJ, Davis R, George RK, Rivers B, Wingerd MA, Torres M, Dent A, 2013. Systemic absorption of mitomycin-C when used in refractive surgery. J. Cataract Refract. Surg. 39, 193–196. [DOI] [PubMed] [Google Scholar]
- Dawson DG, Edelhauser HF, Grossniklaus HE, 2005. Long-term histopathologic findings in human corneal wounds after refractive surgical procedures. Am. J [DOI] [PubMed] [Google Scholar]
- de Benito-Llopis L, Cañadas P, Drake P, Hernández-Verdejo JL, Teus MA, 2012. Keratocyte density 3 months, 15 months, and 3 years after corneal surface ablation with mitomycin C. Am. J. Ophthalmol 153, 17–23.e1. [DOI] [PubMed] [Google Scholar]
- de Benito-Llopis L, Teus MA, Ortega M, 2007. Effect of mitomycin-C on the corneal endothelium during excimer laser surface ablation. J. Cataract Refract. Surg. 33, 1009–1013. [DOI] [PubMed] [Google Scholar]
- de Oliveira RC, Wilson SE, 2020. Fibrocytes, wound healing, and corneal fibrosis. Invest. Ophthalmol. Vis. Sci 61, 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakonis VF, Pallikaris A, Kymionis GD, Markomanolakis MM, 2007. Alterations in endothelial cell density after photorefractive keratectomy with adjuvant mitomycin. Am. J. Ophthalmol 144, 99–103. [DOI] [PubMed] [Google Scholar]
- Erie JC, 2003. Corneal wound healing after photorefractive keratectomy: a 3-year confocal microscopy study. Trans. Am. Ophthalmol. Soc 101, 293–333. [PMC free article] [PubMed] [Google Scholar]
- Erie JC, McLaren JW, Hodge DO, Bourne WM, 2005. Recovery of corneal subbasal nerve density after PRK and LASIK. Am. J. Ophthalmol 140, 1059–1064. [DOI] [PubMed] [Google Scholar]
- Erie JC, Patel SV, McLaren JW, Hodge DO, Bourne WM, 2003. Keratocyte density in the human cornea after photorefractive keratectomy. Arch. Ophthalmol. (Chicago, Ill. 1960) 121, 770–776. [DOI] [PubMed] [Google Scholar]
- Farahi A, Hashemi H, Mehravaran S, 2013. The effects of mitomycin C on tear function after photorefractive keratectomy: a contralateral comparative study. J. Refract. Surg 29, 260–264. [DOI] [PubMed] [Google Scholar]
- Fini ME, 1999. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog. Retin. Eye Res. 18, 529–551. [DOI] [PubMed] [Google Scholar]
- Galm U, Hager MH, Van Lanen SG, Ju J, Thorson JS, Shen B, 2005. Antitumor antibiotics: bleomycin, enediynes, and mitomycin. Chem. Rev 105, 739–758. [DOI] [PubMed] [Google Scholar]
- Gambato C, Ghirlando A, Moretto E, Busato F, Midena E, 2005. Mitomycin C modulation of corneal wound healing after photorefractive keratectomy in highly myopic eyes. Ophthalmology. 112, 208–18 [DOI] [PubMed] [Google Scholar]
- Gambato C, Miotto S, Cortese M, Ghirlando A, Lazzarini D, Midena E, 2011. Mitomycin C-assisted photorefractive keratectomy in high myopia: a long-term safety study. Cornea 30, 641–645. [DOI] [PubMed] [Google Scholar]
- Gatinel D, Racine L, Hoang-Xuan T, 2007. Contribution of the corneal epithelium to anterior corneal topography in patients having myopic photorefractive keratectomy. J. Cataract Refract. Surg. 33, 1860–1865. [DOI] [PubMed] [Google Scholar]
- Gauthier CA, Holden BA, Epstein D, Tengroth B, Fagerholm P, Hamberg-Nystrom H, 1997. Factors affecting epithelial hyperplasia after photorefractive keratectomy. J. Cataract Refract. Surg. 23, 1042–1050. [DOI] [PubMed] [Google Scholar]
- Gharaee H, Zarei-Ghanavati S, Alizadeh R, Abrishami M, 2018. Endothelial cell changes after photorefractive keratectomy with graded usage of mitomycin C. Int. Ophthalmol 38, 1211–1217. [DOI] [PubMed] [Google Scholar]
- Gipson IK, 1990. Corneal epithelial and stromal reactions to excimer laser photorefractive keratectomy. I. Concerns regarding the response of the corneal epithelium to excimer laser ablation. Arch. Ophthalmol. (Chicago, Ill. 1960). [DOI] [PubMed] [Google Scholar]
- Goldsberry DH, Epstein RJ, Majmudar PA, Epstein RH, Dennis RF, Holley G, Edelhauser HF, 2007. Effect of mitomycin C on the corneal endothelium when used for corneal subepithelial haze prophylaxis following photorefractive keratectomy. J. Refract. Surg 23, 724–727. [DOI] [PubMed] [Google Scholar]
- Hashemi H, Salimi Y, Pir P, Asgari S, 2017. Photorefractive Keratectomy With Mitomycin-C for High Myopia: Three Year Follow-Up Results. Acta Med. Iran. 55, 42–48. [PubMed] [Google Scholar]
- Hashemi H, Taheri SMR, Fotouhi A, Kheiltash A, 2004. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy in high myopia: a prospective clinical study. BMC Ophthalmol. 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi M, Amiri MA, Tabatabaee M, Ayatollahi A, 2016. The results of photorefractive keratectomy with Mitomycin-C in myopia correction after 5 years. Pakistan J. Med. Sci. 32, 225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell JR, Birk DE, 2010. The molecular basis of corneal transparency. Exp. Eye Res. 91, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata T, Hoshi T, Kanamori K, Matsumae A, Sano Y, Shima T, Sugawara R, 1956. Mitomycin, a new antibiotic from Streptomyces. I. J. Antibiot. (Tokyo). 9, 141–146. [PubMed] [Google Scholar]
- Hayashi S, Ishimoto S, Wu GS, Wee WR, Rao NA, McDonnell PJ, 1997. Oxygen free radical damage in the cornea after excimer laser therapy. Br. J. Ophthalmol 81, 141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Call MK, Chikama T, Liu H, Carlson EC, Sun Y, Pearlman E, Funderburgh JL, Babcock G, Liu C-Y, Ohashi Y, Kao WW-Y, 2010. Lumican is required for neutrophil extravasation following corneal injury and wound healing. J. Cell Sci. 123, 2987–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helena MC, Baerveldt F, Kim WJ, Wilson SE, 1998. Keratocyte apoptosis after corneal surgery. Invest. Ophthalmol. Vis. Sci 39, 276–283. [PubMed] [Google Scholar]
- Hersh PS, Stulting RD, Steinert RF, Waring GO 3rd, Thompson KP, O’Connell M, Doney K, Schein OD, 1997. Results of phase III excimer laser photorefractive keratectomy for myopia. The Summit PRK Study Group. Ophthalmology 104, 1535–1553. [DOI] [PubMed] [Google Scholar]
- Hindman HB, DeMagistris M, Callan C, McDaniel T, Bubel T, Huxlin KR, 2019. Impact of topical anti-fibrotics on corneal nerve regeneration in vivo. Exp. Eye Res. 181, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeister EM, Bishop FM, Kaupp SE, Schallhorn SC, 2013. Randomized dose-response analysis of mitomycin-C to prevent haze after photorefractive keratectomy for high myopia. J. Cataract Refract. Surg. 39, 1358–1365. [DOI] [PubMed] [Google Scholar]
- Hong J-W, Liu JJ, Lee J-S, Mohan RR, Mohan RR, Woods DJ, He Y-G, Wilson SE, 2001. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Invest. Ophthalmol. Vis. Sci 42, 2795–2803. [PubMed] [Google Scholar]
- Iyer VN, Szybalski W, 1964. Mitomycins and porfiromycin: Chemical mechanism of activation and cross-linking of DNA. Science 145, 55–58. [DOI] [PubMed] [Google Scholar]
- Iyer VN, Szybalski W, 1963. A molecular mechanism of mitomycin action: Linking of complementary DNA strands. Proc. Natl. Acad. Sci. U. S. A 50, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon K-I, Hindman HB, Bubel T, McDaniel T, DeMagistris M, Callan C, Huxlin KR, 2018. Corneal myofibroblasts inhibit regenerating nerves during wound healing. Sci. Rep 8, 12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Nien CJ, Vasiliou V, Brown DJ, 2012. Quiescent keratocytes fail to repair MMC induced DNA damage leading to the long-term inhibition of myofibroblast differentiation and wound healing. Mol Vis. 18, 1828–39. [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Ho-Chang J, 2003. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp. Eye Res. 77, 581–592. [DOI] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J, 1999. The cellular basis of corneal transparency: evidence for “corneal crystallins”. J. Cell Sci. 112 (Pt 5), 613–622. [DOI] [PubMed] [Google Scholar]
- Kaiserman I, Sadi N, Mimouni M, Sela T, Munzer G, Levartovsky S, 2017. Corneal breakthrough haze after photorefractive keratectomy with mitomycin C: Incidence and Risk Factors. Cornea 36, 961–966. [DOI] [PubMed] [Google Scholar]
- Kampmeier J, Radt B, Birngruber R, Brinkmann R, 2000. Thermal and biomechanical parameters of porcine cornea. Cornea 19, 355–363. [DOI] [PubMed] [Google Scholar]
- Kawase K, Matsushita H, Yamamoto T, Kitazawa Y, 1992. Mitomycin concentration in rabbit and human ocular tissues after topical administration. Ophthalmology 99, 203–207. [DOI] [PubMed] [Google Scholar]
- Kennedy KA, Rockwell S, Sartorelli AC, 1980a. Preferential activation of mitomycin C to cytotoxic metabolites by hypoxic tumor cells. Cancer Res. 40, 2356–2360. [PubMed] [Google Scholar]
- Kennedy KA, Teicher BA, Rockwell S, Sartorelli AC, 1980b. The hypoxic tumor cell: a target for selective cancer chemotherapy. Biochem. Pharmacol 29, 1–8. [DOI] [PubMed] [Google Scholar]
- Khoury JM, Farah T, El-Haibi CP, Noureddin BN, 2007. Corneal light shield as a delivery system for standardized application of mitomycin C in excimer surface ablation. J. Refract. Surg 23, 716–719. [DOI] [PubMed] [Google Scholar]
- Kim T-I, Pak JH, Lee SY, Tchah H, 2004. Mitomycin C-induced reduction of keratocytes and fibroblasts after photorefractive keratectomy. Invest. Ophthalmol. Vis. Sci 45, 2978–2984. [DOI] [PubMed] [Google Scholar]
- Kim T, Tchah H, Lee S, Sung K, Cho BJ, Kook MS, 2003. Apoptosis in keratocytes caused by mitomycin C. Invest. Ophthalmol. Vis. Sci 44, 1912–1917. [DOI] [PubMed] [Google Scholar]
- Kim WJ, Mohan RR, Mohan RR, Wilson SE, 1999. Effect of PDGF, IL-1alpha, and BMP2/4 on corneal fibroblast chemotaxis: expression of the platelet-derived growth factor system in the cornea. Invest. Ophthalmol. Vis. Sci 40, 1364–1372. [PubMed] [Google Scholar]
- Kremer I, Ehrenberg M, Levinger S, 2012. Delayed epithelial healing following photorefractive keratectomy with mitomycin C treatment. Acta Ophthalmol. 90, 271–276. [DOI] [PubMed] [Google Scholar]
- Kymionis GD, Diakonis VF, Charisis S, Pallikaris AI, Bouzoukis DI, Yoo SH, Naoumidi I, Tsilimbaris MK, 2008. Effects of topical mitomycin C on the ciliary body and intraocular pressure after PRK: an experimental study. J. Refract. Surg 24, 633–638. [DOI] [PubMed] [Google Scholar]
- Kymionis GD, Diakonis VF, Panagopoulou SI, Grentzelos MA, Kazakos DC, Tzatzarakis MN, Tsatsakis AM, Pallikaris AI, 2009. Mitomycin C aqueous humor concentration after photorefractive keratectomy: an experimental study. Eur. J. Ophthalmol 19, 738–742. [DOI] [PubMed] [Google Scholar]
- Lassance L, Marino GK, Medeiros CS, Thangavadivel S, Wilson SE, 2018. Fibrocyte migration, differentiation and apoptosis during the corneal wound healing response to injury. Exp. Eye Res. 170, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leccisotti A, 2009. Mitomycin-C in hyperopic photorefractive keratectomy. J. Cataract Refract. Surg. 35, 682–687. [DOI] [PubMed] [Google Scholar]
- Leccisotti A, 2008. Mitomycin C in photorefractive keratectomy: effect on epithelialization and predictability. Cornea 27, 288–291. [DOI] [PubMed] [Google Scholar]
- Lee DH, Chung HS, Jeon YC, Boo SD, Yoon YD, Kim JG, 2005. Photorefractive keratectomy with intraoperative mitomycin-C application. J. Cataract Refract. Surg. 31, 2293–2298. [DOI] [PubMed] [Google Scholar]
- Legeais JM, Mayer F, Saragoussi JJ, Abenhaim A, Renard G, 1997. [The optical power of the corneal epithelium. In vivo evaluation]. J. Fr. Ophtalmol 20, 207–212. [PubMed] [Google Scholar]
- Lohmann CP, Reischl U, Marshall J, 1999. Regression and epithelial hyperplasia after myopic photorefractive keratectomy in a human cornea. J. Cataract Refract. Surg. 25, 712–715. [DOI] [PubMed] [Google Scholar]
- Mack M, 2018. Inflammation and fibrosis. Matrix Biol. 68–69, 106–121. [DOI] [PubMed] [Google Scholar]
- Majmudar PA, Forstot SL, Dennis RF, Nirankari VS, Damiano RE, Brenart R, Epstein RJ, 2000. Topical mitomycin-C for subepithelial fibrosis after refractive corneal surgery. Ophthalmology 107, 89–94. [DOI] [PubMed] [Google Scholar]
- Majmudar PA, Schallhorn SC, Cason JB, Donaldson KE, Kymionis GD, Shtein RM, Verity SM, Farjo AA, 2015. Mitomycin-C in corneal surface excimer laser ablation techniques: a report by the American Academy of Ophthalmology. Ophthalmology 122, 1085–1095. [DOI] [PubMed] [Google Scholar]
- Marino GK, Santhiago MR, Santhanam A, Torricelli AAM, Wilson SE, 2017. Regeneration of defective epithelial basement membrane and restoration of corneal transparency after photorefractive keratectomy. J. Refract. Surg 33, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino GK, Santhiago MR, Torricelli AAM, Santhanam A, Wilson SE, 2016. Corneal molecular and cellular biology for the refractive surgeon: The critical role of the epithelial basement membrane. J. Refract. Surg 32, 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott ML, Wang J, Shin DH, 1994. Mitomycin and the human corneal endothelium. Arch. Ophthalmol. (Chicago, 1ll. 1960) 112, 533–537. [DOI] [PubMed] [Google Scholar]
- Medeiros CS, Marino GK, Lassance L, Thangavadivel S, Santhiago MR, Wilson SE, 2018a. The Impact of Photorefractive Keratectomy and Mitomycin C on Corneal Nerves and Their Regeneration. J. Refract. Surg 34, 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros CS, Marino GK, Santhiago MR, Wilson SE, 2018b. The Corneal Basement Membranes and Stromal Fibrosis. Invest. Ophthalmol. Vis. Sci. 59, 4044–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midena E, Gambato C, Miotto S, Cortese M, Salvi R, Ghirlando A, 2007. Long-term effects on corneal keratocytes of mitomycin C during photorefractive keratectomy: a randomized contralateral eye confocal microscopy study. J. Refract. Surg 23, S1011–4. [DOI] [PubMed] [Google Scholar]
- Mietz H, Addicks K, Bloch W, Krieglstein GK, 1996. Long-term intraocular toxic effects of topical mitomycin C in rabbits. J. Glaucoma 5, 325–333. [PubMed] [Google Scholar]
- Mladenov E, Tsaneva I, Anachkova B, 2007. Activation of the S phase DNA damage checkpoint by mitomycin C. J. Cell. Physiol 211, 468–476. [DOI] [PubMed] [Google Scholar]
- Mohammadi S-F, Ashrafi E, Norouzi N, Abdolahinia T, Mir-AbouTalebi M, Jabbarvand M, 2014. Effects of mitomycin-C on tear film, corneal biomechanics, and surface irregularity in mild to moderate myopic surface ablation: preliminary results. J. Cataract Refract. Surg. 40, 937–942. [DOI] [PubMed] [Google Scholar]
- Mohammadi SF, Abdolahinia T, Ashrafi E, Heydari S, Jamali S, 2019. Long-term effects of mitomycin-C on residual aberration and optical quality after photorefractive keratectomy in eyes with low to moderate myopia. J. Cataract Refract. Surg. 45, 1351–1352. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AEK, Choi R, Hong J, Lee J, Mohan RR, Ambrosio RJ, Zieske JD, Wilson SE, 2003. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp. Eye Res. 76, 71–87. [DOI] [PubMed] [Google Scholar]
- Morales AJ, Zadok D, Mora-Retana R, Martinez-Gama E, Robledo NE, Chayet AS, 2006. Intraoperative mitomycin and corneal endothelium after photorefractive keratectomy. Am. J. Ophthalmol 142, 400–404. [DOI] [PubMed] [Google Scholar]
- Nassaralla BA, McLeod SD, Nassaralla JJJ, 2007. Prophylactic mitomycin C to inhibit corneal haze after photorefractive keratectomy for residual myopia following radial keratotomy. J. Refract. Surg 23, 226–232. [DOI] [PubMed] [Google Scholar]
- Nassiri Nader, Farahangiz S, Rahnavardi M, Rahmani L, Nassiri Nariman, 2008. Corneal endothelial cell injury induced by mitomycin-C in photorefractive keratectomy: nonrandomized controlled trial. J. Cataract Refract. Surg. 34, 902–908. [DOI] [PubMed] [Google Scholar]
- Netto MV, Barreto JJ, Santo R, Bechara S, Kara-Jose N, Wilson SE, 2008. Synergistic effect of ethanol and mitomycin C on corneal stroma. J. Refract. Surg 24, 626–632. [DOI] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE, 2006a. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp. Eye Res. 82, 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Gupta PC, Wilson SE, 2006b. Effect of prophylactic and therapeutic mitomycin C on corneal apoptosis, cellular proliferation, haze, and long-term keratocyte density in rabbits. J. Refract. Surg 22, 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogrebniak HW, Matthews W, Pass HI, 1991. Chemotherapy amplifies production of tumor necrosis factor. Surgery 110, 231–237. [PubMed] [Google Scholar]
- Pritsos CA, Sartorelli AC, 1986. Generation of reactive oxygen radicals through bioactivation of mitomycin antibiotics. Cancer Res. 46, 3528–3532. [PubMed] [Google Scholar]
- Rajan MS, Jaycock P, O’Brart D, Nystrom HH, Marshall J, 2004. A long-term study of photorefractive keratectomy; 12-year follow-up. Ophthalmology 111, 1813–1824. [DOI] [PubMed] [Google Scholar]
- Rajan MS, O’Brart DPS, Patmore A, Marshall J, 2006. Cellular effects of mitomycin-C on human corneas after photorefractive keratectomy. J. Cataract Refract. Surg. 32, 1741–1747. [DOI] [PubMed] [Google Scholar]
- Razmjoo H, Kooshanmehr MR, Peyman A, Kor Z, Mohammadesmaeil E, 2013. Comparison of standard and low dose intraoperative mitomycin C in prevention of corneal hazeafter photorefractive keratectomy. Int. J. Prev. Med 4, 204–207. [PMC free article] [PubMed] [Google Scholar]
- Roh DS, Cook AL, Rhee SS, Joshi A, Kowalski R, Dhaliwal DK, Funderburgh JL, 2008. DNA cross-linking, double-strand breaks, and apoptosis in corneal endothelial cells after a single exposure to mitomycin C. Invest. Ophthalmol. Vis. Sci 49, 4837–4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D, Beall HD, Siegel D, Traver RD, Gustafson DL, 1996. Enzymology of bioreductive drug activation. Br. J. Cancer. Suppl 27, S1–8. [PMC free article] [PubMed] [Google Scholar]
- Sadeghi HM, Seitz B, Hayashi S, LaBree L, McDonnell PJ, 1998. In vitro effects of mitomycin-C on human keratocytes. J. Refract. Surg 14, 534–540. [DOI] [PubMed] [Google Scholar]
- Salomao MQ, Wilson SE, 2009. Corneal molecular and cellular biology update for the refractive surgeon. J. Refract. Surg 25, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhiago MR, Netto MV, Wilson SE, 2012. Mitomycin C: biological effects and use in refractive surgery. Cornea 31, 311–321. [DOI] [PubMed] [Google Scholar]
- Schipper I, Suppelt C, Gebbers JO, 1997. Mitomycin C reduces scar formation after excimer laser (193 nm) photorefractive keratectomy in rabbits. Eye (Lond). 11 ( Pt 5), 649–655. [DOI] [PubMed] [Google Scholar]
- Seow HA, Penketh PG, Baumann RP, Sartorelli AC, 2004. Bioactivation and resistance to mitomycin C. Methods Enzymol. 382, 221–233. [DOI] [PubMed] [Google Scholar]
- Shalchi Z, O’Brart DPS, McDonald RJ, Patel P, Archer TJ, Marshall J, 2015. Eighteen-year follow-up of excimer laser photorefractive keratectomy. J. Cataract Refract. Surg. 41, 23–32. [DOI] [PubMed] [Google Scholar]
- Shojaei A, Ramezanzadeh M, Soleyman-Jahi S, Almasi-Nasrabadi M, Rezazadeh P, Eslani M, 2013. Short-time mitomycin-C application during photorefractive keratectomy in patients with low myopia. J. Cataract Refract. Surg. 39, 197–203. [DOI] [PubMed] [Google Scholar]
- Song J-S, Kim J-H, Yang M, Sul D, Kim H-M, 2007. Mitomycin-C concentration in cornea and aqueous humor and apoptosis in the stroma after topical mitomycin-C application: effects of mitomycin-C application time and concentration. Cornea 26, 461–467. [DOI] [PubMed] [Google Scholar]
- Sorkin N, Rosenblatt A, Smadja D, Cohen E, Santhiago MR, Varssano D, Yatziv Y, 2019. Early refractive and clinical outcomes of high-myopic photorefractive keratectomy as an alternative to LASIK surgery in eyes with high preoperative percentage of tissue altered. J. Ophthalmol 2019, 6513143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ME, Waltz KM, Beurerman RW, Ghosn CR, Mantras CE, Nicolson M, Assouline M, Stern KL, Wheeler LA, 1995. Effect of platelet-derived growth factor on rabbit corneal wound healing. Wound repair Regen. Off. Publ. Wound Heal. Soc. [and] Eur. Tissue Repair Soc. 3, 59–65. [DOI] [PubMed] [Google Scholar]
- Sy ME, Zhang L, Yeroushalmi A, Huang D, Hamilton DR, 2014. Effect of mitomycin-C on the variance in refractive outcomes after photorefractive keratectomy. J. Cataract Refract. Surg. 40, 1980–1984. [DOI] [PubMed] [Google Scholar]
- Szybalski W, Iyer VN, 1964. Crosslinking of DNA by enzymatically or chemically activated mitomycins and porfiromycins, bifunctionally “alkylating” antibiotics. Fed. Proc 23, 946–957. [PubMed] [Google Scholar]
- Talamo JH, Gollamudi S, Green WR, De La Cruz Z, Filatov V, Stark WJ, 1991. Modulation of corneal wound healing after excimer laser keratomileusis using topical mitomycin C and steroids. Arch. Ophthalmol. (Chicago, Ill. 1960) 109, 1141–1146. [DOI] [PubMed] [Google Scholar]
- Thomas KE, Brunstetter T, Rogers S, Sheridan MV, 2008. Astigmatism: risk factor for postoperative corneal haze in conventional myopic photorefractive keratectomy. J. Cataract Refract. Surg. 34, 2068–2072. [DOI] [PubMed] [Google Scholar]
- Thornton I, Xu M, Krueger RR, 2008. Comparison of standard (0.02%) and low dose (0.002%) mitomycin C in the prevention of corneal haze following surface ablation for myopia. J. Refract. Surg 24, S68–76. [DOI] [PubMed] [Google Scholar]
- Tomasz M, Palom Y, 1997. The mitomycin bioreductive antitumor agents: cross-linking and alkylation of DNA as the molecular basis of their activity. Pharmacol. Ther 76, 73–87. [DOI] [PubMed] [Google Scholar]
- Torres RM, Merayo-Lloves J, Daya SM, Blanco-Mezquita JT, Espinosa M, Nozal MJ, Bernal JL, Bernal J, 2006. Presence of mitomycin-C in the anterior chamber after photorefractive keratectomy. J. Cataract Refract. Surg. 32, 67–71. [DOI] [PubMed] [Google Scholar]
- Torricelli AAM, Santhanam A, Wu J, Singh V, Wilson SE, 2016. The corneal fibrosis response to epithelial-stromal injury. Exp. Eye Res. 142, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AAM, Singh V, Agrawal V, Santhiago MR, Wilson SE, 2013. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest. Ophth. Vis. Sci 54, 4026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J, Pinedo HM, 1990. Mitomycin C: mechanism of action, usefulness and limitations. Anticancer. Drugs 1, 5–13. [PubMed] [Google Scholar]
- Vestergaard AH, Hjortdal JØ, Ivarsen A, Work K, Grauslund J, Sjølie AK, 2013. Long-term outcomes of photorefractive keratectomy for low to high myopia: 13 to 19 years of follow-up. J. Refract. Surg 29, 312–319. [DOI] [PubMed] [Google Scholar]
- Virasch VV, Majmudar PA, Epstein RJ, Vaidya NS, Dennis RF, 2010. Reduced application time for prophylactic mitomycin C in photorefractive keratectomy. Ophthalmology 117, 885–889. [DOI] [PubMed] [Google Scholar]
- West-Mays JA, Dwivedi DJ, 2006. The keratocyte: corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol. 38, 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2020a. Corneal wound healing. Exp. Eye Res. 108089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2020b. Biology of keratorefractive surgery—PRK, PTK, LASIK, SMILE, inlays and other refractive surgeries. Exp Eye Res. 10.1016/j.exer.2020.108136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2020c. Corneal myofibroblasts and fibrosis. Exp Eye Res, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2019. Coordinated modulation of corneal scarring by the epithelial basement membrane and Descemet’s basement membrane. J. Refract. Surg 35, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2012. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Exp. Eye Res. 99, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, He YG, Weng J, Li Q, McDowall AW, Vital M, Chwang EL, 1996. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp. Eye Res. 62, 325–327. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Medeiros CS, Santhiago MR, 2018. Pathophysiology of corneal scarring in persistent epithelial defects after PRK and other corneal injuries, J. Ref. Surg 34, 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Mohan RR, Ambrósio RJ, Hong J, Lee J, 2001. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retin. Eye Res. 20, 625–637. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Netto M, Ambrósio RJ, 2003. Corneal cells: chatty in development, homeostasis, wound healing, and disease. Am. J. Ophthalmol 136, 530–536. [DOI] [PubMed] [Google Scholar]
- Woo JE, Park WC, Yoo YH, Kim SW, 2014. The efficacy of co-treatment with suberoylanilide hydroxamic acid and mitomycin C on corneal scarring after therapeutic keratectomy: an animal study. Curr. Eye Res. 39, 348–358. [DOI] [PubMed] [Google Scholar]
- Wu KY, Hong SJ, Huang HT, Lin CP, Chen CW, 1999. Toxic effects of mitomycin-C on cultured corneal keratocytes and endothelial cells. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther 15, 401–411. [DOI] [PubMed] [Google Scholar]
- Zare M, Jafarinasab M-R, Feizi S, Zamani M, 2011. The effect of mitomycin-C on corneal endothelial cells after photorefractive keratectomy. J. Ophthalmic Vis. Res. 6, 8–12. [PMC free article] [PubMed] [Google Scholar]
- Zhao L-Q, Wei R-L, Ma X-Y, Zhu H, 2008. Effect of intraoperative mitomycin-C on healthy corneal endothelium after laser-assisted subepithelial keratectomy. J. Cataract Refract. Surg 34, 1715–1719. [DOI] [PubMed] [Google Scholar]


