Fig. 1.
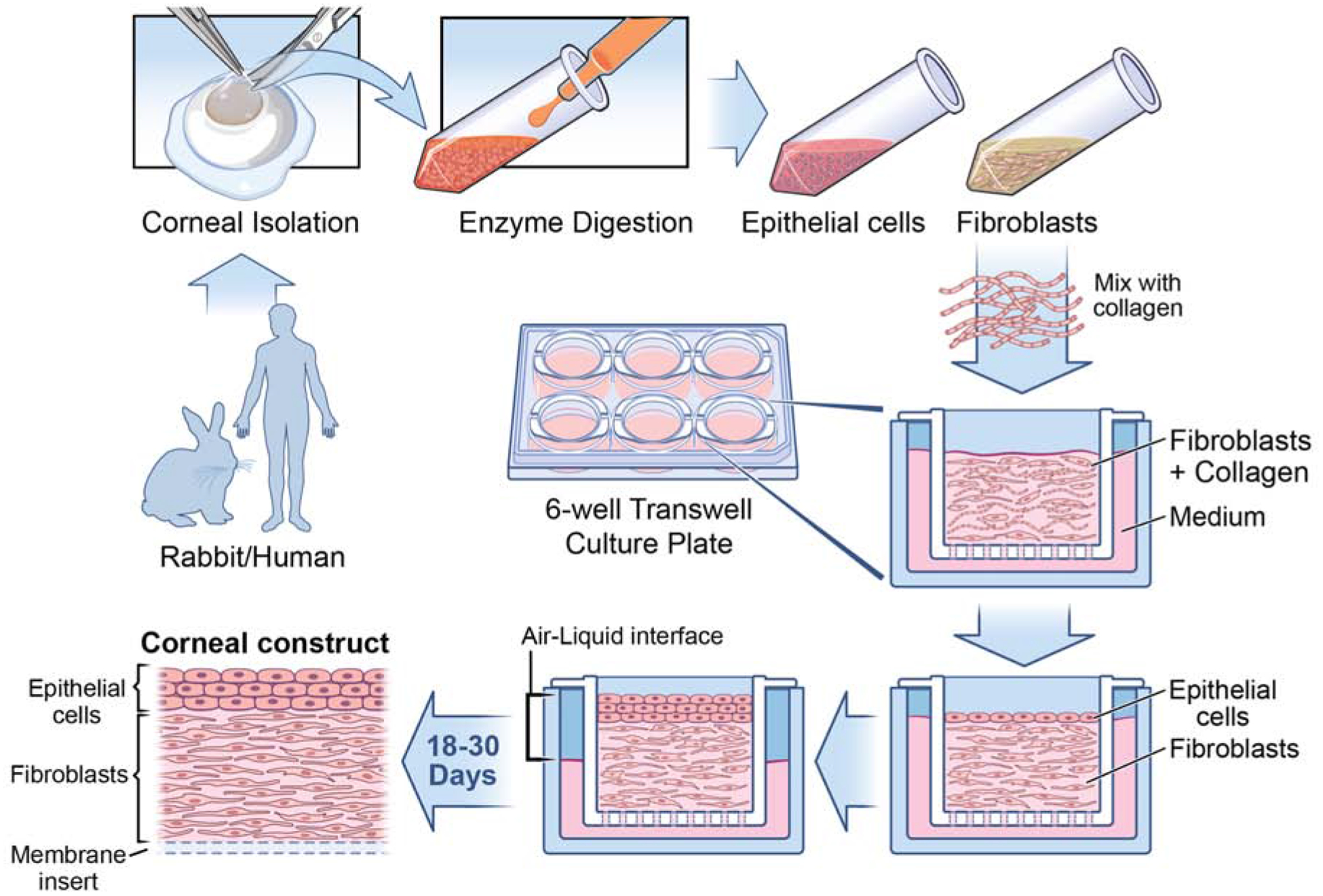
Corneal fibroblast and epithelial 3D organotypic culture workflow. Rabbit/Human corneas subjected to epithelium scrape and descemetorhexis, isolating epithelial cells and stromal layer. Corneal keratocytes are obtained through overnight incubation of stromal layer with collagenase medium (50 mg of collagenase and 25 mg of Hyaluronidase in 50 ml HBSS). The following day, the desired cell type is collected via brief centrifugation and cultured in fibroblast medium containing Fibroblast growth factor-2 (40ng/ml), which promotes fibroblast differentiation. Once the required cell count is reached, fibroblasts are seeded along with collagen onto the insert of a 6 well tissue culture tray and the medium is added to both inside and outside of the insert. After 5 days of culture, epithelial cells are seeded above the collagen layer. From this stage onwards, the medium is added only to the bottom of the insert so as to maintain the cells in both air and liquid phase, which is vital in culturing cells in 3D. The cells are maintained in the air-liquid interface for about 18–30 days to get the 3D corneal construct containing fibroblasts embedded in collagen with epithelial cells on the top. It is interesting to observe the presence of multiple layers of epithelial cells above the newly deposited EBM, similar to what is observed in vivo.
