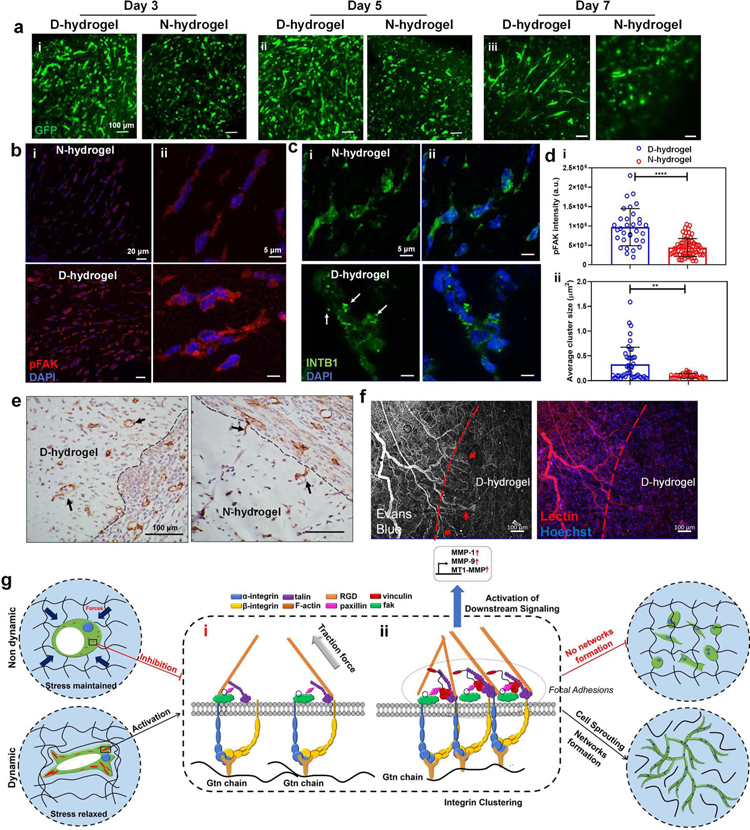
Fig. 7. Dynamic networks accelerate vasculogenesis in vivo and proposed molecular pathway of ECFCs in response to dynamic networks.
See also Figures S6 and S7. (a) GFP-ECFC-loaded D- and N-hydrogels were directly implanted subcutaneously in nude mice and retrieved after (i) day 3, (ii) day 5, and (iii) day 7 (n = 3). Representative confocal images show GFP-ECFC (in green) of the corresponding extracted hydrogels. (b) FAK activation is increased in ECFC-loaded D-hydrogels compared with N-hydrogels in vivo on day 5 indicated by pFAK signal intensity (pFAK in red, nuclei in blue) scale bars are 20 µm (i) and 5 µm (ii) (c) integrin cluster size is larger in ECFC-loaded D-hydrogels (β1-integrin in green some indicated by arrows, nuclei in blue) in vivo on day 5 scale bars are 20 µm (i) and 5 µm (ii). (d) quantification of pFAK signal intensity (i) and β1-integrin cluster size. (e) Representative histological images of CD31+ vessels infiltrating into acellular hydrogels in D-hydrogels compared to individual cells invading into the edge of N-hydrogels (indicated by arrows). Scale bars are 100 µm (f) Vessels, labeled with lectin, infiltrating into D-hydrogels were perfused (indicated by arrows) with Evans blue dye injected intravenously. Scale bars are 100 µm. Significance levels were set at **p ≤ 0.01, ***p ≤ 0.001 and ****p ≤ 0.0001. (g) Hydrogels with dynamic networks enable the rapid formation of FA in a stiffness-independent manner. Contrarily, static covalent hydrogels do not facilitate the formation of FA, leading to an abrogation of vascular morphogenesis. In both systems, ECFCs interact with the hydrogel binding sites, leading to vacuole and lumen formation. (i) The rigidity of the non dynamic matrix prevents the formation of integrin clusters via cell contractility inhibition; (ii) In the dynamic matrix, integrin β1 interaction with RGD binding sites of the Gtn leads to the recruitment of FAK and other FA proteins. In a second step, pMLC mediated actin contractility leads to the formation of larger integrin clusters. Integrin clustering and the recruitment of vinculin to the FAs leads to the formation of larger, stable FAs. These FAs allow for robust downstream signaling and further FAK activation. Activated FAK then further contributes to cell contraction and integrin expression promoting the formation of larger integrin clusters and taking in part in robust downstream signaling. The activation of FAK leads to the upregulation of the MT1-MMP and MMP-1, MMP-9, resulting in matrix degradation and remodeling, allowing the progression of ECFC vasculogenesis.