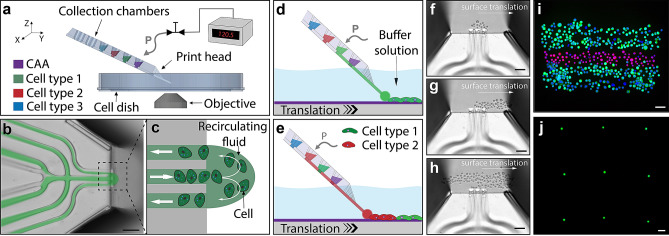
Figure 1.
Introduction to the direct cell bioprinting approach, highlighting the key components. (a–c) illustrates the core concept of printing cells from a recirculating fluid flow. (a) Schematic overview of the bioprinting setup consisting of; a micro-positioner controlled printhead, an automated substrate positioner, a computer controlled pneumatic interface, and a microscopy imaging system. CAA refers to cell adhesion agent. (b) An overlay image combining fluorescence and brightfield modalities, demonstrating the fluidic printhead and the hydrodynamically confined flow. (c) An illustration of the fluidic printhead tip, presenting the flow path of cells within the confined circulating fluid. (d,e) illustrate the method of pattern formation using multiple cell types. (d) Positioning the printhead in proximity to a substrate surface within a buffer solution, whilst maintaining a confined fluid flow, enables cells to interact and attach to the substrate within a localised region at the tip of the printhead. Translating the surface relative to the printhead allows the deposited cells to be patterned. (e) Changing which solution within the printhead is actively being pressurised, allows numerous loaded cell types to be printed, without the need to replace or reload the printhead. (f–h) Experimental brightfield images demonstrate the formation of a structured pattern composed of alternating stripes of HaCaT and A431 cells onto a petri dish surface immersed in DMEM growth media. (f) presents HaCaT cells within the confined flow at the beginning of a print. (g) shows the first stripe of the pattern being printed. (h) presents the image mid-way through second pass of the HaCaT cell stripe. (i) presents a three-line structure composed of alternating printed stripes of HaCaT and A431 cells, labelled with cytotracker-green and cytotracker-red respectively. This structure was post-print nuclei stained with Hoechst 33342 and fixed with paraformaldehyde. A demonstration of the printing precision is shown in (j), whereby a single cell array was constructed using A431 cells. The scale bars represent 100 µm.