Figure 5.
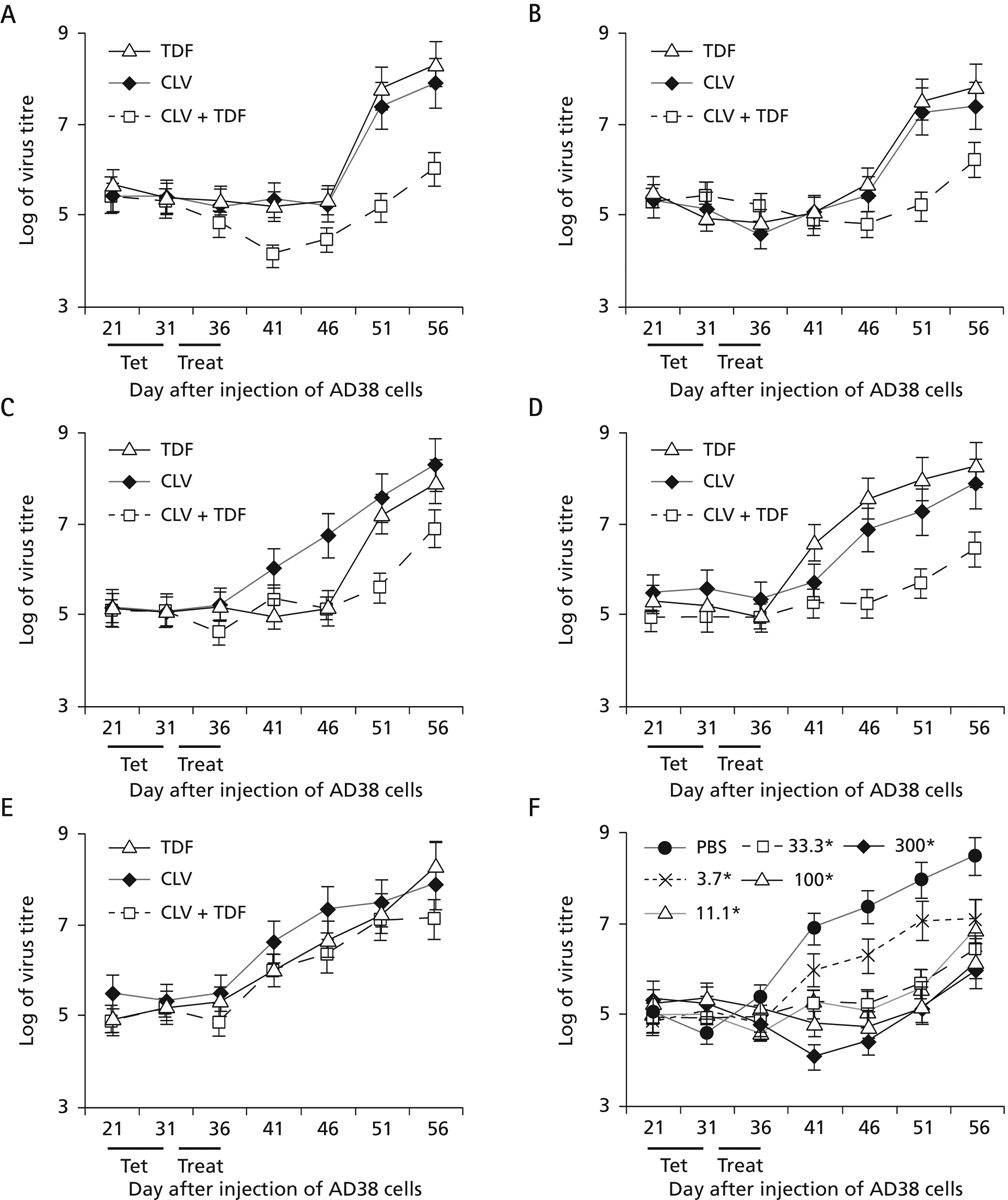
Treatment of mice with different doses of TDF, CLV or with TDF + CLV combination therapy
The protocol outlined in Figure 1 was followed. Five mice per group were treated with tenofovir dipivoxil fumarate (TDF), clevudine (CLV) or with TDF + CLV combination therapy. Doses were administered at (A) 300 mg/kg per day, (B) 100 mg/kg per day, (C) 33.3 mg/kg per day, (D) 11.1 mg/kg per day or (E) 3.7 mg/kg per day for 6 consecutive days (days 31–36). Mice were then bled over the next 30 days as indicated. (F) Curves reflecting virus DNA titres in serum resulting from different concentrations of combination therapy shown in panels A–E were compared with each other and to phosphate buffered saline (PBS)-fed mice. *All concentrations in F are measured as mg/kg per day. Treat, treatment period.
