Figure 1. Changes in Metabolism and Epigenetics during Hematopoietic Stem Cell (HSC) Activation and Aging.
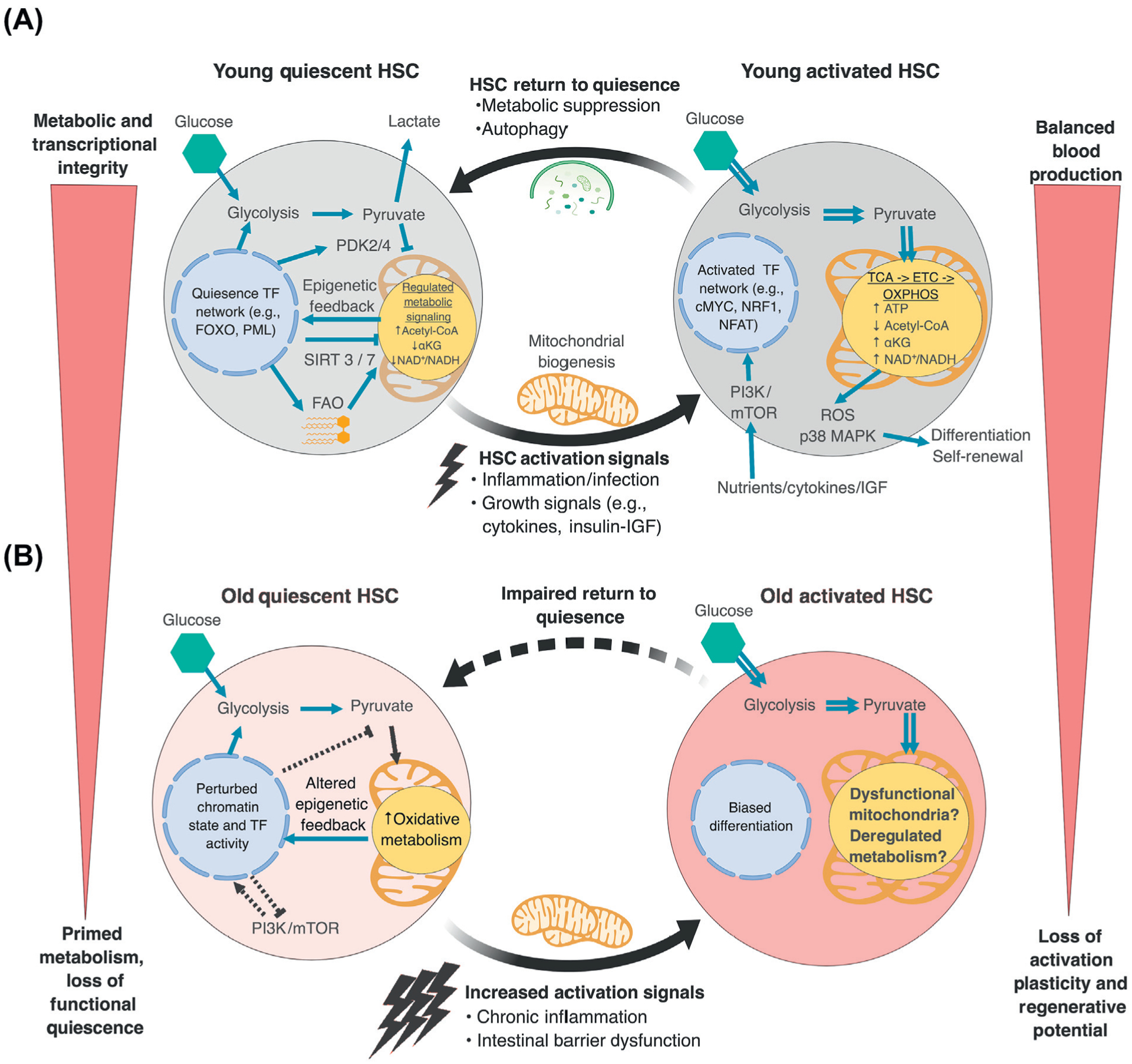
(A) In young HSCs, quiescence is maintained by a hardwired transcriptional regulatory network promoting anaerobic glycolysis and limiting the entry of pyruvate into the tricarboxylic acid (TCA) cycle. Low aerobic flux and fatty acid oxidation (FAO) also provide important substrates that participate in metabolic and epigenetic regulation and help to maintain quiescence. In response to activation signals, HSCs engage oxidative phosphorylation (OXPHOS), which is coupled with mitochondrial biogenesis, mTOR activation, and transcriptional reprogramming. This metabolic switch promotes reactive oxygen species (ROS) production and MAPK activation and stimulates HSC differentiation. When the needs of the hematopoietic system are met, HSCs engage autophagy to clear metabolically activated mitochondria and suppress oxidative metabolism to return to quiescence. (B) With age, old HSCs face challenges with the suppression of basal oxidative metabolism and maintenance of effective quiescence as a result of altered transcriptional regulatory networks and changes to cell-surface signaling, including exposure to higher levels of proinflammatory cytokines. This promotes stem cell exhaustion and blood aging phenotypes. ETC, electron transport chain.
