Abstract
Background and aims
Diabetes is one of the most common comorbidities, and it is associated with poorer outcomes in patients with coronavirus disease 2019 (COVID-19). Preliminary findings showed that mortality was reduced in those who consume metformin compared to those who did not, and given its low cost and widespread availability; metformin is an attractive and potential agent to mitigate excessive risk in diabetic populations.
Methods
Several medical databases (Pubmed, EuropePMC, EBSCOhost, Proquest, Cochrane library) and two health-science preprint servers (preprint.org and Medrxiv) were systematically searched for relevant literature.
Results
Nine studies with 10,233 subjects were included in the qualitative and quantitative synthesis. Meta-analysis showed that metformin is associated with lower mortality in pooled non-adjusted model (OR 0.45 [0.25, 0.81], p = 0.008; I2: 63.9%, p = 0.026) and pooled adjusted model (OR 0.64 [0.43, 0.97], p = 0.035; I2: 52.1%, p = 0.064).
Conclusion
The analysis showed that metformin consumption was associated with lower mortality. Randomized controlled trials are needed to confirm this finding.
Keywords: COVID-19, SARS-CoV-2, Diabetes melitus, Metformin, Mortality
Highlights
-
•
Diabetes is unequivocally associated with poorer outcomes regarding COVID-19 disease.
-
•
Preliminary findings showed that mortality was lowered in those who consume metformin vs. who did not.
-
•
Thus, metformin is an attractive and potential regimen for mitigating excessive risk in diabetic populations.
-
•
Our meta-analysis confirmed that in hospitalized COVID-19 patients, metformin consumption was associated with lower mortality.
1. Introduction
Amid the worst pandemic of the modern human history, which started as an outbreak of an unknown respiratory disease in Wuhan, China on December 31, 2019, caused by a severe acute respiratory syndrome virus 2 (SARS-CoV-2), and subsequently identified as a coronavirus disease of 2019 (COVID-19), curative measures are yet to be seen [[1], [2], [3]]. At the time this paper was written, the total COVID-19 cases throughout the world have surpassed 28 million people, and the number of death cases is reaching one million marks, exactly 922,525 people [4].
COVID-19 has been known to affect patients with comorbidities disproportionately, and one of the most prevalent comorbidities throughout the world is diabetes mellitus. A systematic review has previously identified that diabetes is significantly associated with poor outcomes in COVID-19 patients [5]. Early evidence has shown that metformin, a biguanide oral hypoglycemic agent and one of the widely used anti-diabetic drugs, was associated with lower mortality [6]. Thus, in light of paucity regarding high-quality data of metformin consumption and mortality in COVID-19 patients, we want to explore the state-of-the-art evidence regarding this critical topic through this systematic review and meta-analysis.
2. Methods
Several medical databases (Pubmed, EuropePMC, EBSCOhost, Proquest, Cochrane library) and two health-science preprint servers (preprint.org and Medrxiv) were systematically searched for relevant literature. The keywords constitute of (“Metformin” OR “Biguanides”) AND (“COVID-19″ OR “SARS-CoV-2″ OR “NCov-2019″ OR “Novel Coronavirus”) AND (“Mortality”). The inclusion criteria for this systematic review were articles of primarily observational studies (cross-sectional, case-control, retrospective/prospective cohort studies) that reported the odds ratios (ORs) or hazard ratios (HRs) in adjusted and non-adjusted forms regarding patients who consume metformin vs who did not. The exclusion criteria were review articles, non-research letters, communications, and commentaries; studies with samples <20; case reports and small case series; research in pediatric populations (younger than 17 years of age). An English language only restriction will be applied, and we will include articles available from January 1, 2020.
Additionally, hand searches will also be performed to incorporate relevant studies not covered by the database search. The systematic search was finalized on September 5, 2020. Two independent authors performed the systematic search, and third and fourth persons adjudicated discrepancies. This systematic search is reported per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). This study is registered in the PROSPERO, international prospective register of systematic reviews (ID: CRD42020203383).
2.1. Data extraction
A standardized form was utilized to help data extraction, which was performed by two researchers. The form contains the following information: the author’s name, country, study design, number of subjects, publication status (published/preprint), sex, age, comorbidities, lactic acidosis, and HbA1C. To assess the included studies’ risk of bias, the Newcastle-Ottawa Score (NOS) was used by two independent authors, and a third author adjudicated any differences.
2.2. Statistical analysis
Review Manager 5.4 [7]. and STATA version 16.0 (StataCorp LLC, Texas, US) were used for meta-analysis. Pooled estimates in the form of odds ratios (ORs; adjusted and non-adjusted forms) and its 95% confidence interval was used to characterize the association between metformin consumption and mortality by using the generic inverse variance method. Moreover, a random-effects model was assigned to account for interstudy variability, regardless of the heterogeneity. The significance of two-tailed p values was set at ⩽0.05. Heterogeneity across studies was assessed using the inconsistency index (I2) with a value above 50% or p < 0.10 indicates significant heterogeneity, whereas I2 <25% is considered low heterogeneity. Finally, an inverted funnel-plot analysis was used to detect any publication bias qualitatively.
3. Results
3.1. Study selection and characteristics
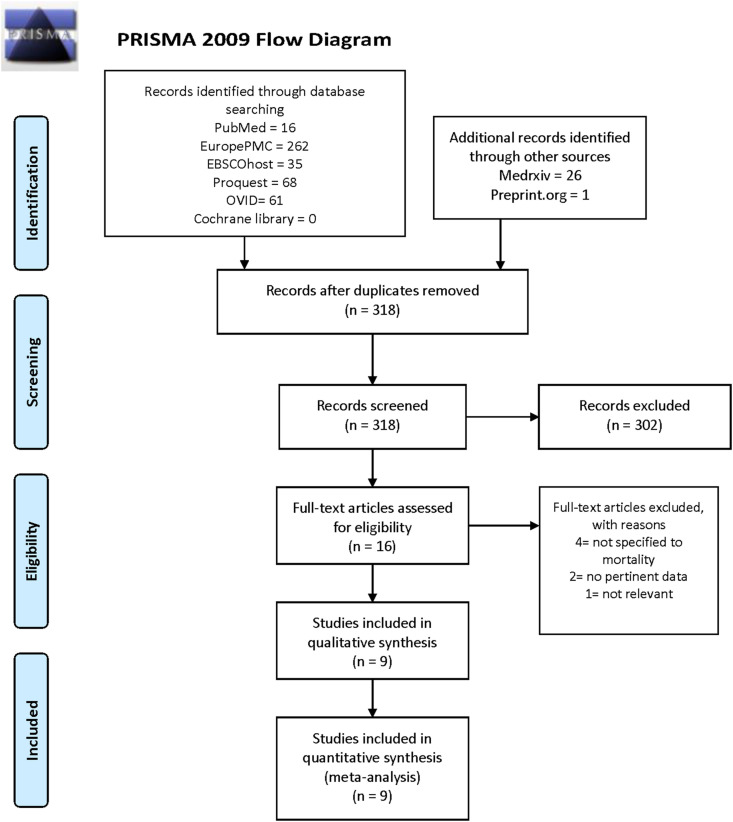
Initial search from several databases and preprint servers yielded 496 potential articles, of which 27 were preprint articles. After excluding duplicates, 318 articles remained for the title and abstract screening. Subsequently, a total of 315 articles were excluded, with the remaining articles underwent full-text eligibility assessment. Finally, out of 16 studies assessed for full-text eligibility, nine studies with 10,233 subjects were included in the qualitative and quantitative synthesis [6,[8], [9], [10], [11], [12], [13], [14], [15]].
(Fig. 1 , Table 1 ) Out of 9 studies included, 4 of them were preprint studies. The mean NOS of the included studies was 8.55±0.52, indicating high-quality studies.
Fig. 1.
PRISMA flow diagram.
Table 1.
Characteristics of the included studies.
| No. | Author | Country | Study Design | Preprint | Subjects | Male | Overall age Mean ± SD /Median (IQR) |
Mortality (Metformin vs non Metformin) | OR | CKD | Lactic Acidosis | HbA1C (%) | Newcastle Ottawa Scale |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abu-Jamous et al. [8] | UK | Retrospective cohort | Yes | 411 | 4 vs 94 | 0.19 (0.05–0.70) | 97 | N/A | N/A | 9 | ||
| 2 | Cariou et al. [9] | France | Prospective cohort | No | 1317 | 855 (64.9%) | 69.8 ± 13.0 | N/A | 0.59 (0.42–0.84) | 355/1066 (33.3%) | 8.1 ± 1.9 | 8 | |
| 3 | Bramante et al. [15] | USA | Retrospective cohort | Yes | 6256 | 2954 (47.2%); 1204 vs 1750 | 73.0 (66.0–80.0) vs 76.0 (67.0–84.0) | 394 (17.8%) vs 791 (21.3%) | OR 0.802 (0.701, 0.917) | 14 (0.6%) vs 297 (7.6%) | N/A | N/A | 9 |
| 4 | Chen et al. [10] | USA | Retrospective study | No | 120 | N/A | 66.0 (60.5–73.5) | 4 (9.30%) vs 15 (19.48%) | N/A | 16 | N/A | 7.70 (6.90–9.13) vs 8.40 (7.35–10.65) | 8 |
| 5 | Crouse et al. [6] | USA | Retrospective cohort | Yes | 239 | 121 (50.6%) | 8 (19%) vs 34 (81%) | OR 0.38 (0.17, 0.87) p = 0.0221 | N/A | N/A | 7.3 (1.3) vs 6.6 (2.1) | 8 | |
| 6 | Kim et al. [13] | South Korea | Retrospective Observational study | No | 235 | 106 (45.1) | 68.3 ± 11.9 | N/A | 0.36 (0.10–1.23) p = 0.10 | 18 (7.7%) | N/A | 7.7 ± 1.79 | 9 |
| 7 | Luo et al. [14] | China | Retrospective study | No | 283; 104 vs 179 | 53 (51.0) vs 103 (57.5) | 63.0 (55.8–68.3) vs 65.0 (57.5–71.0) | 3 (2.9%) vs 22 (12.3%) | 4.36 ((1.22–15.59) p = 0.02 | 1 (1.0) vs 3 (1.7) | N/A | N/A | 9 |
| 8 | Philipose et al. [12] | UK | Retrospective cohort | Yes | 159 | 158 vs 119 | N/A | 45 (22.6) | 1.39 (0.84–2.16) | N/A | N/A | 50.5 (23.5, 0–108, 68) | 8 |
| 9 | Cheng et al. [11] | China | Retrospective cohort | No | 1213; 678 vs 535 | 632 (52.1%); 365 (53.8%) vs 267 (49.9%) | 63.0 (56.0–69.0); 62.0 (55.0–68.0) vs 64.0 (58.0–70.0) | N/A | 1.65 (0.71,3.86) p = 0.27 | 16 (2.4%) vs 14 (2.6%) | 12 (1.77%) vs 0.75% | 8.1 (7.0%–9.9%) vs 7.6, (6.7%–8.9%) | 9 |
3.2. Mortality
Metformin is associated with lower mortality in pooled non-adjusted model (OR 0.45 [0.25, 0.81], p = 0.008; I2: 63.9%, p = 0.026) [Fig. 2 ] and pooled adjusted model (OR 0.64 [0.43, 0.97], p = 0.035; I2: 52.1%, p = 0.064) [Fig. 3 ].
Fig. 2.
Metformin consumption and mortality in hospitalized adult COVID-19 patients; Pooled non-adjusted model.
Fig. 3.
Metfomin consumption and mortality in hospitalized adult COVID-19 patients; Pooled adjusted model.
3.3. Publication bias
Harbord’s test and Egger’s test indicated the presence of small-study effects for unadjusted (p = 0.008) and adjusted model (p = 0.006), respectively. Funnel-plot was symmetrical, indicating no publication bias [Fig. 4 ].
Fig. 4.
Inverted funnel plot analysis
The inverted funnel plot analysis showed a symmetrical funnel plot, which indicates no publication bias.
4. Discussion
This meta-analysis showed that metformin was associated with lower mortality in patients with COVID-19 in pooled unadjusted and adjusted models.
Obesity and diabetes mellitus (DM) are independent, significant risk factors for mortality in coronavirus disease 2019 (COVID-19) [5,16]. The presence of excessive body mass index (BMI) and type 2 DM in patients with severe COVID-19 is quite prevalent among other comorbidities, including advanced age, hypertension, and heart, lung, kidney, liver, and cerebrovascular diseases [[17], [18], [19], [20], [21], [22], [23], [24], [25]]. In addition, physical inactivity or sedentariness commonly observed in these subjects contributed to impaired immune function and increased morbidity and mortality in COVID-19, regardless of the presence of insulin resistance [26,27].
Generally known as an anti-diabetic medication, metformin was initially discovered as an anti-malarial agent and later introduced as an anti-influenza drug with one of the side effects of lowering blood glucose level. The use of metformin as an oral hypoglycemic drug was approved by the Food and Drug Administration (FDA) in 1995 and has since become one of the most frequently prescribed anti-diabetic medications worldwide with further therapeutic potential. Its efficacy has been suggested to enhance insulin sensitivity, promote weight loss, decrease cytokines formation by macrophages, inhibit viral activity, and treat a wide variety of conditions, such as metabolic syndrome, cardiovascular diseases, autoimmune diseases, neurological diseases, and cancer [28,29].
It is thought that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) adheres to and utilizes human angiotensin-converting enzyme 2 (ACE2) receptors as entry points to invade target cells, including pneumocytes, cardiac epithelial cells, podocytes and proximal straight tubular cells, and liver endothelial cells [24,30]. The hypothetical impacts of metformin in COVID-19 are assumed to be mediated by ACE2 upregulation which could explain the epidemiological association between diabetes and COVID-19 [31]. This effect was also seen in COVID-19 patients who received synergistic treatment with angiotensin II receptor blockers (ARB) or angiotensin-converting enzyme inhibitors (ACEI) [32].
Obese and diabetic individuals have systemic, chronic, low-grade inflammation which makes them vulnerable to infections, including viral pneumonia, with poorer outcomes [16]. Cytokine storms have been postulated to cause worsening outcomes in patients infected with SARS-CoV-2. In severe and critically ill COVID-19 patients, the exaggerated immune responses and overproduction of pro-inflammatory cytokines cause a state of hyperinflammation or cytokine storm which is responsible to the development of serious complications, long-term lung damage and fibrosis, functional disability, reduced quality of life, and finally death [24].
In the context of COVID-19, metformin could be a promising option not only for managing glucose levels, but also used as a potential agent in host-directed therapy to reduce mortality [14]. The use of metformin has been found to reduce the levels of pro-inflammatory cytokines, such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α, and increase the concentrations of anti-inflammatory cytokines, such as IL-10. It has been found that metformin has sex-specific immunomodulatory actions related to cytokines, in which the beneficial actions in females were more significant than in males [15]. Metformin use stimulates the formation of M2 macrophages and T-regulatory and CD8 memory T cells while limiting the expression of genes encoding cytokines and chemokines that ultimately enhance immune response and diminish inflammation. This agent also positively influences microbiota composition and ultimately minimize inflammation. Moreover, metformin consumption can promote autophagy, which contributes to the activation of the host’s innate and adaptive immune responses, controls excessive inflammation, and contains and destroys pathogens [14].
Metformin also induces the activation of 5′-adenosine monophosphate-activated protein kinase (AMPK), which potentially improving ACE2 expression, phosphorylation, and stability and ultimately brings significant downstream impact on SARS-CoV-2 [31]. This activation is mediated by liver kinase B1 (LKB1) and attenuation of phosphatidylinositol 3-kinase (PI3K) and protein kinase B 9 (AKT) activation via phosphorylation of insulin receptor substrate 1 (IRS-1) leading to inhibition of the mammalian target of rapamycin (mTOR) signalling cascade. Since metformin blocks the PI3K/AKT/mTOR pathway, which plays a crucial part in the pathogenesis of influenza as observed in Middle East respiratory syndrome coronavirus (MERS-CoV) infection, it is plausible to elaborate the possibility of using this agent as a host-directed therapy for COVID-19 [29]. Furthermore, this medication helps in improving neutrophil to lymphocyte ratio, boosting protection against oxidative stress, stabilizing mast cell, enhancing endothelial function, and limiting thrombosis [15]. These favourable effects of metformin may elucidate the findings of lower complications and consequently reduced mortality in COVID-19.
A study had found that SARS-CoV-2 invades endocrine tissues that express ACE2 and damages β-cell in pancreatic islets, resulting in acute, transient T2DM [33]. Therefore, optimal control of T2DM, for both chronic and transient cases, could help in treating COVID-19 patients comprehensively [29]. The glucose-lowering effect of metformin is achieved by improving insulin sensitivity, modulating glycogen synthesis, and consequently decreasing hepatic glucose production. It is therefore well tolerated and rarely causes hypoglycemia any individual irrespective of their diabetes status. In addition, the risk of lactic acidosis is minimal in patients with impaired kidney and liver function [14]. Thus, metformin should be considered as a host-directed therapy for COVID-19 patients in all BMI categories, whether they have diabetes or not, considering the excellent safety profile and availability of metformin.
4.1. Limitations
From 9 studies included in our systematic review, four were preprint studies. Nevertheless, these studies are funded by the United States’ national institute of health (n = 2) and the United Kingdom’s national health service (n = 1), which add credibility to these preprint articles. Furthermore, all but one study were retrospective in nature and can introduce unnecessary bias to our findings. Thus, prospective studies are needed to confirm our systematic review.
5. Conclusion
Our meta-analysis showed that whether in non-adjusted and adjusted models, metformin consumption was associated with lower mortality. Nevertheless, the presence of small study effects necessitate larger studies, especially well-designed randomized control trials.
CRediT author statement
Antonia Anna Lukito: Conceptualization, Writing – Original Draft, Project Administration. Raymond Pranata: Formal Analysis, Writing – Review & Editing. Joshua Henrina: Formal Analysis, Data Curation, Writing – Original Draft. Michael Anthonius Lim: Writing – Original Draft. Sherly Lawrensia: Data Curation, Writing – Original Draft.Ketut Suastika: Writing – Review & Editing.
Data availability
The data used to support the findings of this study are included within the article.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. The corresponding author (AAL) can be contacted for more information.
Code availability
Not applicable.
Conflicts of interest
The authors declare that they have no competing interests.
Funding
None.
Acknowledgments
None.
Abbreviations
- ACE2
Angiotensin Converting Enzyme 2
- AKT
Protein kinase B 9
- AMPK
5′-adenosine monophosphate-activated protein kinase
- ARB
Angiotensin Receptor Blocker
- BMI
body mass index
- COVID-19
Coronavirus Disease 2019
- DM
Diabetes Mellitus
- FDA
Food and Drug Administration
- HR
Hazard ratio
- IL:
Interleukin
- IRS-1
Insulin receptor substrate 1
- LKB1
Liver kinase B1
- MERS-CoV
Middle East respiratory syndrome coronavirus
- mTOR
mammalian Target of Rapamycin
- NOS
Newcastle-Ottawa Scale
- OR
Odds Ratio
- PI3K
Phosphatidylinositol 3-kinase
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- TNF-α
Tumor necrosis factor-α
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020:1–13. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopkins University Johns. Johns Hopkins University (JHU); 2020. COVID-19 dashboard by the center for systems science and engineering (CSSE) at.https://coronavirus.jhu.edu/map.html accessed. [Google Scholar]
- 5.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression: diabetes and COVID-19. Diabetes Metab Syndr Clin Res Rev. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crouse A., Grimes T., Li P., Might M., Ovalle F., Shalev A. Metformin use IS associated with reduced mortality IN a diverse population with COVID-19 and diabetes. MedRxiv Prepr Serv Heal Sci. 2020 doi: 10.1101/2020.07.29.20164020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cochrance. Review manager (RevMan) 2020. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman accessed. [Google Scholar]
- 8.Abu-Jamous B., Anisimovich A., Baxter J., Mackillop L., Vizcaychipi M., McCarthy A. 2020. Associations of comorbidities and medications with COVID-19 outcome: a retrospective analysis of real-world evidence data. [DOI] [Google Scholar]
- 9.Bertrand C., Samy H., Matthieu W., Matthieu P., Al-Salameh A., Ingrid A. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43:1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 11.Cheng X., Liu YM., Li H., Zhang X., Lei F., Qin JJ. Cell Metab; 2020. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and pre-existing type 2 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philipose Z., Smati N., Wong C.S.J., Aspey K., Mendall M.A. Obesity, old age and frailty are the true risk factors for COVID-19 mortality and not chronic disease or ethnicity in Croydon. MedRxiv. 2020:2020. doi: 10.1101/2020.08.12.20156257. 08.12.20156257-2020.08.12.20156257. [DOI] [Google Scholar]
- 13.Kim M.K., Jeon J.H., Kim S.W., Moon J.S., Cho N.H., Han E. The clinical characteristics and outcomes of patients with moderate-to-severe coronavirus disease 2019 infection and diabetes in daegu, South Korea. Diabetes Metab J. 2020;44:602–613. doi: 10.4093/dmj.2020.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo P., Qiu L., Liu Y., Liu X.-L., Zheng J.-L., Xue H.-Y. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. 2020;103:69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bramante C., Ingraham N., Murray T., Marmor S., Hoversten S., Gronski J. Observational study of metformin and risk of mortality in patients hospitalized with covid-19. MedRxiv Prepr Serv Heal Sci. 2020 doi: 10.1101/2020.06.19.20135095. 2020.06.19.20135095. [DOI] [Google Scholar]
- 16.Pranata R., Lim M.A., Yonas E., Vania R., Lukito A.A., Siswanto B.B. Body mass index and outcome in patients with COVID-19: a dose-response meta-analysis. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim M.A., Huang I., Yonas E., Vania R., Pranata R. A wave of non-communicable diseases following the COVID-19 pandemic. Diabetes Metab Syndr Clin Res Rev. 2020;14:979–980. doi: 10.1016/j.dsx.2020.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. JRAAS - J Renin-Angiotensin-Aldosterone Syst. 2020:1–11. doi: 10.1177/14703203209268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yonas E., Alwi I., Pranata R., Huang I., Lim M.A., Gutierrez E.J. Effect of heart failure on the outcome of COVID-19 - a meta analysis and systematic review. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pranata R., Lim M.A., Yonas E., Siswanto B.B., Meyer M. Out-of-hospital cardiac arrest prognosis during the COVID-19 pandemic. Intern Emerg Med. 2020 doi: 10.1007/s11739-020-02428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pranata R., Tondas A.E., Huang I., Lim M.A., Siswanto B.B., Meyer M. Potential role of telemedicine in solving ST-segment elevation dilemmas in remote areas during the COVID-19 pandemic. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim M.A., Pranata R. Coronavirus disease 2019 (COVID-19) markedly increased mortality in patients with hip fracture – a systematic review and meta-analysis. J Clin Orthop Trauma. 2020 doi: 10.1016/j.jcot.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pranata R., Soeroto A.Y., Huang I., Lim M.A., Santoso P. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tubercul Lung Dis. 2020 doi: 10.5588/ijtld.20.0278. [DOI] [PubMed] [Google Scholar]
- 24.Lim M.A., Pranata R., Huang I., Yonas E., Soeroto A.Y., Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Heal Dis. 2020;7 doi: 10.1177/2054358120938573. 2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pranata R., Huang I., Lim M.A., Wahjoepramono P.E.J., July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19 - systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020 doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim M.A., Pranata R. Sports activities during any pandemic lockdown. Ir J Med Sci. 2020;(1971) doi: 10.1007/s11845-020-02300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim M.A. Exercise addiction and COVID-19-associated restrictions. J Ment Health. 2020 doi: 10.1080/09638237.2020.1803234. [DOI] [PubMed] [Google Scholar]
- 28.El-Arabey A.A., Abdalla M. Metformin and COVID-19: a novel deal of an old drug. J Med Virol. 2020 doi: 10.1002/jmv.25958. 10.1002/jmv.25958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma S., Ray A., Sadasivam B. Metformin in COVID-19: a possible role beyond diabetes. Diabetes Res Clin Pract. 2020;164:108183. doi: 10.1016/j.diabres.2020.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620937175. 1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ursini F., Ciaffi J., Landini M.P., Meliconi R. COVID-19 and diabetes: is metformin a friend or foe? Diabetes Res Clin Pract. 2020;164:108167. doi: 10.1016/j.diabres.2020.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pranata R., Permana H., Huang I., Lim M.A., Soetedjo N.N.M., Supriyadi R. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020 doi: 10.1016/j.dsx.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J.-K., Lin S.-S., Ji X.-J., Guo L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.
All data generated or analyzed during this study are included in this published article. The corresponding author (AAL) can be contacted for more information.