Figure 4.
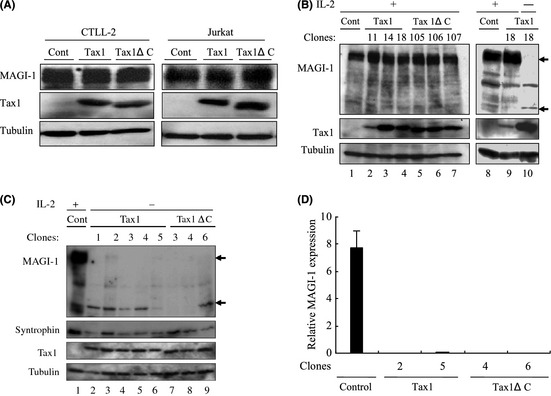
MAGI‐1 downregulation by interleukin (IL)‐2‐independent transformation of T‐cells. (A) CTLL‐2 and Jurkat cells were transfected with lentiviruses encoding Tax1. At 48 h after transfection, cell lysates were prepared and the amounts of MAGI‐1, Tax1 and Tubulin were determined by a Western blotting analysis. (B) CTLL‐2 cells stably expressing Tax1 established as previously described15 were used as IL‐2‐dependent cells (lane 2–9). All the above cells were then transferred to IL‐2 deficient medium and cultured for more than 1 month. Only one Tax1 expressing clone (Tax1–18) survived in the absence of IL‐2 (right panel, lane 10). Cell lysates were prepared from the indicated cells, and protein expression was measured by a Western blotting analysis. (C) CTLL‐2 cells transformed by Tax1 and Tax1ΔC were established as described previously.34 Cell lysates were prepared from these Tax1‐transformed (lanes 2–6), Tax1ΔC‐transformed (lanes 7–9) and parental CTLL‐2 cells (lane 1), and the expressions of MAGI‐1, Tax1, Syntrophin‐β and Tubulin proteins were measured by a Western blotting analysis. (D) A set of clones each transformed by either Tax1 or Tax1ΔC was selected from (C) above, and the relative gene expression of MAGI‐1 was evaluated by the quantitative real‐time polymerase chain reaction (PCR) method. The amounts of MAGI‐1 were normalized to those of glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) expression. The quantitative results are expressed as mean ± standard deviation (SD) of three values per sample. The experiment was independently carried out twice to confirm reproducibility.
