Figure 3.
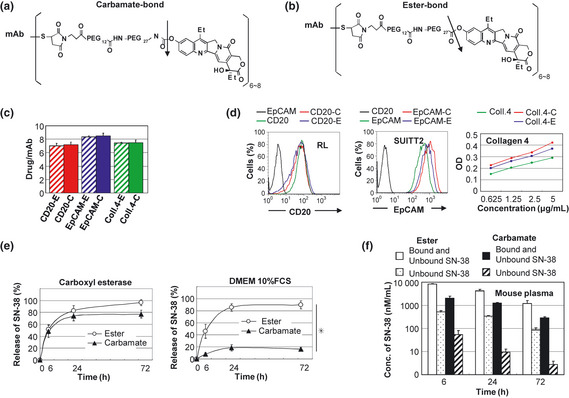
Preparation and characterization of two types of immunoconjugqates‐PEG‐SN‐38 via carbamate‐bond and eser‐bond. (a,b) Drug design of two types of immunoconjugates; monoclonal antibody (mAb) ‐PEG‐SN‐38 via carbamate‐bond (a) and mAb‐PEG‐SN‐38 via ester‐bond (b). One antibody bears six to eight molecules of SN‐38. The arrow indicates the cleavage site for releasing free active SN‐38. (c)The average number of conjugated SN‐38 per one mAb was shown (n = 3). Bar = standard deviation (SD). (d) Antigen‐binding activity of the mAb before and after the conjugation was shown. Anti‐CD 20 and EpCAM mAb were examined by fluorescence‐activated cell sorting (FACS) analysis using RL cells and SUIT2 cells, respectively. Anti‐collagen 4 mAb was examined by enzyme linked immunosorbent assay (ELISA) using purified protein. (e) In vitro release of SN‐38 from two types of immunoconjugates in carboxylesterase‐contained solution (left) and Dulbecco's modified eagle medium (DMEM) 10% fetal calf serum (FCS) (right) (n = 3). Bar standard deviation (SD), *P < 0.05. (f) Concentration of bound and unbound form of SN‐38, and unbound form of SN‐38 from two types of immunoconjugates in the mouse plasma at 6, 24, 72 h after the mice tail vein injection, were shown (n = 3). Concentrations of SN‐38 were determined by high performance liquid chromatography (HPLC). Bar = standard deviation (SD).
