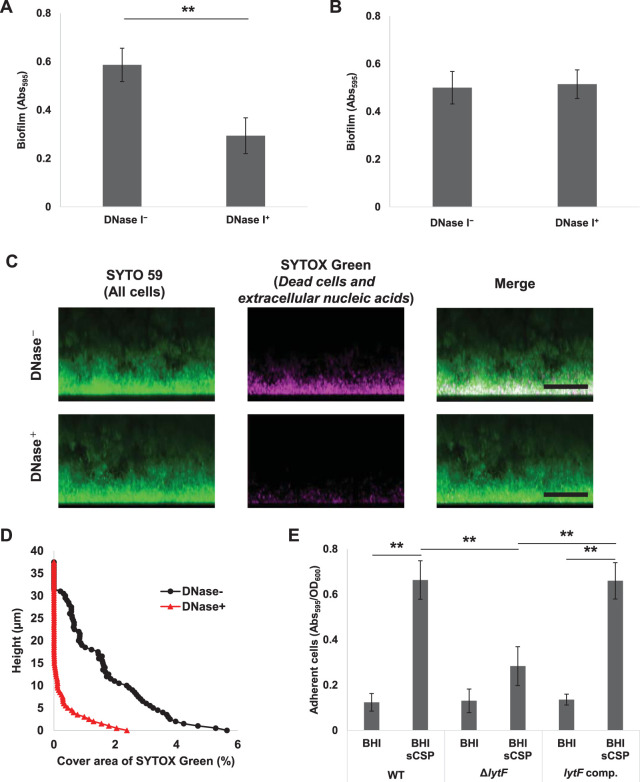
FIG 7.
CSP-induced lytF expression and eDNA production contribute to biofilm adhesion to surfaces. (A) DNase I inhibits biofilm formation. The overnight culture was diluted to an OD600 of 0.05 with BHIs with 1 μM CSP and cultured in an aerobic atmosphere containing 5% CO2 at 37°C for 6 h. DNase I was added to the medium at a concentration of 50 U/ml (DNase+) before the incubation. The biofilms were stained with CV. The absorbance at 595 nm (Abs595) was measured on a plate reader. (B to D) eDNA within the preformed biofilm was degraded by DNase I, but the biofilm structure was maintained. Biofilms were formed in BHIs with sCSP medium in an aerobic atmosphere containing 5% CO2 at 37°C for 6 h. The culture solutions were removed and washed twice with PBS. (B) The biofilms were treated with incubation buffer with or without DNase I (50 U/ml) at 37°C for 6 h. They were then stained with CV and the absorbance at 595 nm (Abs595) was measured on a plate reader. (C) Side views of biofilm cells stained with 5 μM SYTO 59 and 500 μM SYTOX Green for 30 min and observed with inverted CLSM. Z-stacks were acquired at 0.5 μm intervals. SYTO 59 (all cells) and SYTOX Green (eDNA and extracellular nucleic acids) are shown in green and magenta, respectively. The scale bars indicate 20 μm. (D) In each two-dimensional image in panel C, the cover areas showing the fluorescence of SYTOX Green were calculated by ImageJ and Fiji. (E) Quantification of adhered cells to the surface in the WT, ΔlytF, and lytF-complemented (lytF comp.) strains. The cells grown in BHI with sCSP were allowed to adhere to the surface by standing for 6 h at 4°C. Adherent cells were stained with CV for 15 min. The absorbance at 595 nm (Abs595) was measured with a plate reader. The results were standardized by the OD600 value at 6 h of culture. These data indicate the mean ± standard deviations of the results from three independent experiments. The asterisks indicate a significant difference; **, adjusted P < 0.01 (post hoc Bonferroni test).