Figure 1.
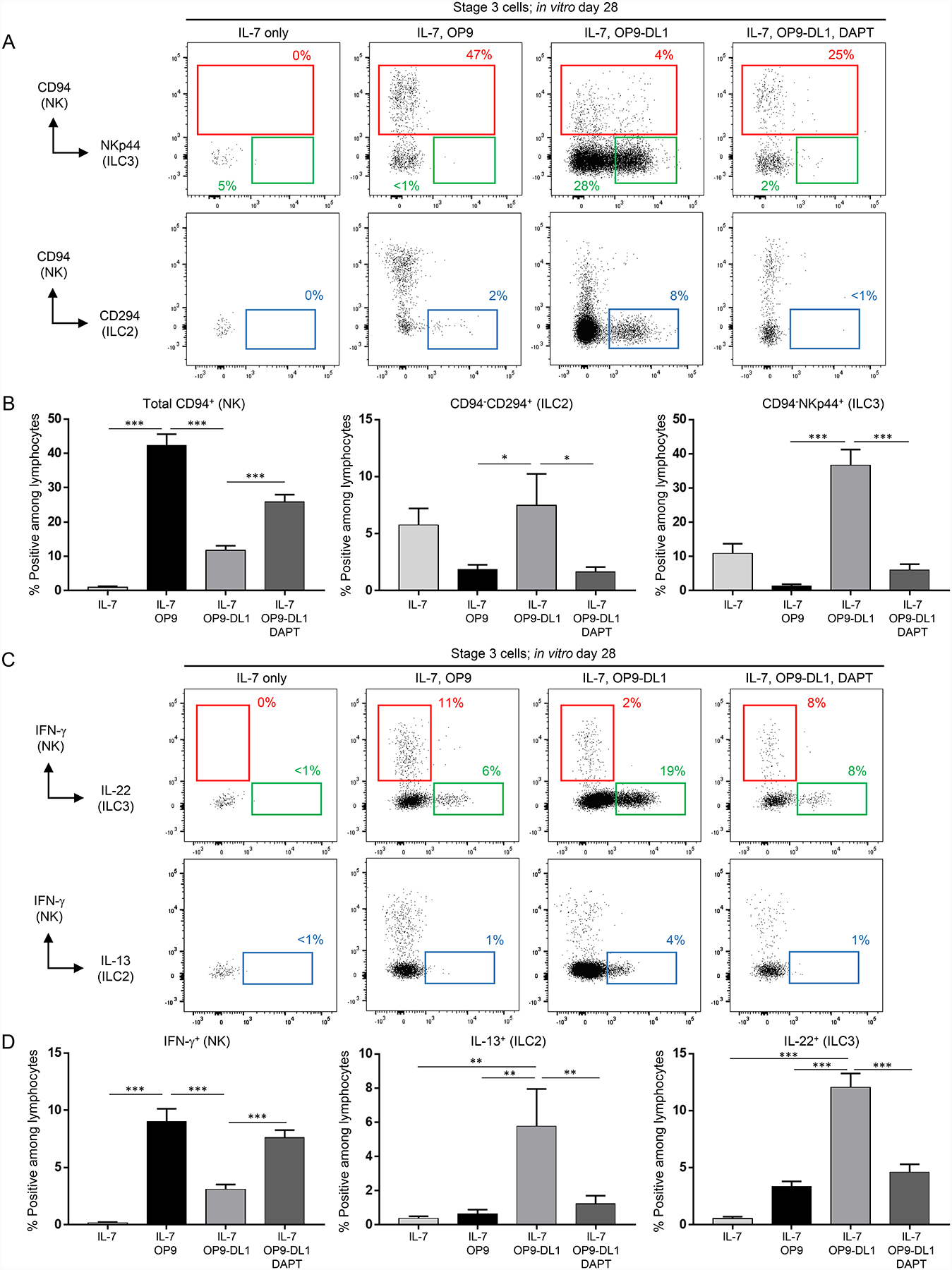
OP9-DL1 stroma support the differentiation of functional ILC2s, ILC3s, and NK cells from stage 3 cells. (A) Representative (n = 16; 5 independent experiments) surface flow cytometry analyses of NK cells (CD94+), ILC2s (CD94−CD294+), and ILC3s (CD94−NKp44+) generated in vitro following 28 day culture of freshly purified tonsil-derived stage 3 cells with recombinant human IL-7 (10 ng/ml for all experiments) alone, IL-7 + OP9 cells, or IL-7 + OP9-DL1 cells and treated with either vehicle control or DAPT (10 μM for all experiments). Dot plots in this and all successive figures are gated on live CD3−CD14−CD45+ lymphocytes unless otherwise labeled. Percentages shown in this and all successive figures represent each population relative to total in vitro-derived live CD3−CD14−CD45+ lymphocytes unless otherwise stated. (B) Quantification of NK cells, ILC2s, and ILC3s generated from stage 3 cells in the conditions described in (A). Data are represented as mean ± SEM. * p < 0.05; *** p < 0.001. (C) Representative (n = 23; 7 independent experiments) intracellular flow cytometry analyses of IFN-γ+, IL-22+, and IL-13+ cells generated in vitro following 28 day culture of freshly purified tonsil-derived stage 3 cells with IL-7 alone, IL-7 + OP9 cells, or IL-7 + OP9-DL1 cells and treated with either vehicle or DAPT. ILCs were stimulated with PMA, ionomycin, and IL-2 (10 ng/ml) for 4 hr prior to analysis of cytokine production. (D) Quantification of IFN-γ+, IL-22+, and IL-13+ cells generated from stage 3 cells in the conditions described in (C). Data are represented as mean ± SEM. ** p < 0.01; *** p < 0.001.
