Fig. 2.
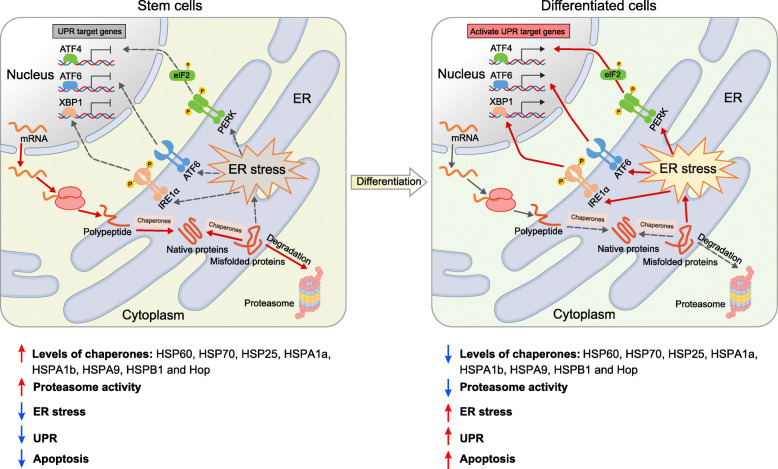
Differences of Unfolded Protein Response (UPR) Networks in Stem Cells and in differentiated Cells. Molecular chaperones facilitate the folding of nascent polypeptides into native protein and the refolding of misfolded proteins as well. If refolding fails, the chaperones deliver misfolded proteins for degradation. High levels of chaperones are present in stem cells, suggesting that stem cells have a greater capacity to assure the proper folding of proteins (Baharvand et al., 2008; Battersby et al., 2007; Saretzki et al., 2004). Similarly, the proteasome activity is also activated in stem cells, regulating the levels of key transcriptional factors and degrading misfolded proteins (Vilchez et al., 2012). Consequently, ER stress, UPR, and apoptosis are kept at a lower level in stem cells. In contrast, during differentiation, the levels of chaperones decrease, whereas ER stress increases (DeLany et al., 2005). The three signal transducers (PERK, IRE1α, and ATF6) of UPR are activated upon the increase of ER stress at the transition from stem cells to mature cell types (Hetz, 2012; Sugiura et al., 2009), suggesting their potential as markers for differentiation (Heijmans et al., 2013)