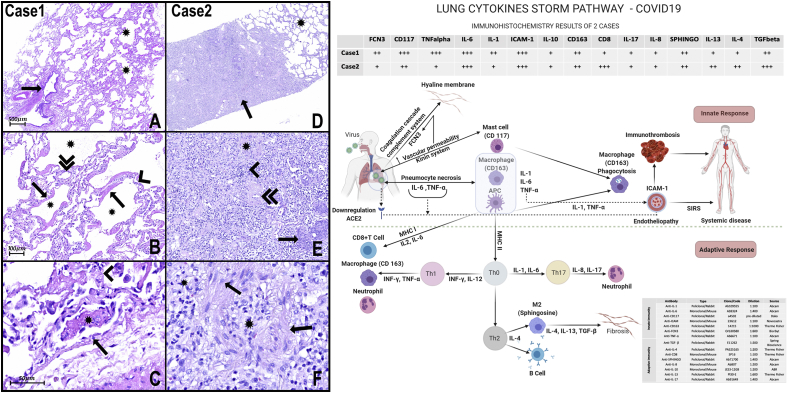
Fig. 1.
H&E Photomicrograph:
A- The panoramic view of the lung sample of case 1 shows one bronchiole (arrow) without histopathological alterations. There are alveoli (asterisks) with multifocal thick hyaline membranes that characterize diffuse alveolar damage (DAD).
B- Numerous alveoli (asterisks) with thick hyaline membranes (arrows) covering delicate alveolar septa (arrowhead). The septal inflammatory infiltrates very mild or almost absent, composed mainly of lymphocytes and some neutrophils, and the septal capillaries are congested (double arrowhead). There is very subtle and focal intra-alveolar edema with slight red cell overflow in some foci. Type II pneumocyte hyperplasia can also be observed.
C- There are some alveolar capillaries and small vessels with signs of endothelial activation (arrow), microthrombi (asterisk), and endotheliitis. Hyaline membranes are recovering delicate septa with very rare lymphocytes and neutrophils (arrowhead).
D- Panoramic view of lung samples of case 2 shows scattered areas of pulmonary fibrosis (arrow) interspersed with areas of histologically preserved lung tissue (asterisk).
E− Lung fibrosis area with collagen and fibroblasts (asterisk) and inflammatory infiltrate (arrowhead). The pulmonary vessels also show signs of endothelial activation and endotheliitis. There is also a thickening of the wall of medium and small arteries (double arrowhead), in addition to foci of squamous metaplasia (arrow).
F- The pulmonary fibrosis areas presented with dense inflammatory infiltrate composed mainly of lymphocytes, macrophages (arrows), and fibroblasts interspersed with areas of intense collagenization (asterisks).
Scheme of Innate and adaptive responses present in cases of COVID-19: In these cases, in response to necrosis of pneumocytes injured by SARS-COV2, we note the massive presence of mast cells (CD117) and macrophages (CD163), capable of secreting cytokines such as TNF-α and interleukins (IL) type 1 and 6, respectively. IL-6 and TNF-α could elevate vascular permeability, promoting exudation of plasma proteins, including ficolin 3 (FCN3). FCN3 and other plasmatic proteins inside the alveoli lumen are responsible for promoting opsonization, activating the complement and the coagulation cascade, and, as observed in the present work, promoting hyaline membranes. The presence of IL-1 and TNF-α, inducing massive expression of ICAM-1 in both cases, can promote the activation of endothelial cells and endotheliitis and may be involved in the progression for systemic inflammatory response syndrome (SIRS). APCs can present the antigens for T lymphocytes, via MHC (main histocompatibility complex), and secreting cytokines that play critical roles in the differentiation of T cells into effector cells. APCs express MHC I molecules and present TCD8+ lymphocytes.
On the other hand, macrophages and APC activate TCD4+ lymphocytes via MHC II, organizing responses classified into subgroups, such as Th1, Th2, Th17, depending on cell differentiation. In case 1 of the present study, a high immunoexpression of CD117 (mast cells) FCN3, TNF-α, IL-1, and Il-6 is observed, suggesting that the inflammatory process is sustained, mainly, through innate immunity. Besides, there is a low number of TCD8+ lymphocytes, suggesting that adaptive immunity is not still adequately stimulated. In contrast, in case 2, in addition to the presence of TCD8+ lymphocytes, there is a marked expression of Sphingosine (M2 macrophages), IL-4, IL1-3, and TGFβ, suggesting the presence of adequately stimulated of adaptive immunity with a tendency to resolve with fibrogenesis. A slight immunoexpression IL-8 and IL-17 is also observed, suggesting low stimulation of the Th17 response and explaining the almost absence of alveolar neutrophil migration. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)