Table 3. Synthesis of 2-Arylidenindane-1,3-diones Promoted by 2-HEAF.
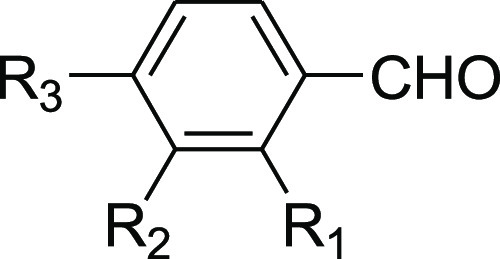
| entry | aldehyde | yield % | mp (°C) |
|---|---|---|---|
| 1 | R1, R2, R3 = H | 98 | 150 |
| 2 | R1, R2 = H; R3 = CH3 | 98 | 150–152 |
| 3 | R1, R2 = H; R3 = OCH3 | 98 | 159 |
| 4 | R1, R2 = H; R3 = NO2 | 98 | 230–232 |
| 5 | R1 = OCH3; R2, R3 = H | 85 | 168 |
| 6 | R1, R2 = OCH3; R3 = H | 98 | 159 |
| 7 | R1, R3 = OCH3; R2 = H | 98 | 204 |
| 8 | R1, R3 = H; R2 = NO2 | 98 | 251–252 |
| 9 | R1, R2 = H; R3 = Cl | 98 | 178 |
| 10 | R1, R3 = Cl; R2 = H | 88 | 194 |
| 11 | 4-(dimethylamino)-benzaldehyde | 98 | 205 |
| 12 | 5-methyl-2-furaldehyde | 98 | 169–170 |
| 13 | 2-thiophencarboxaldehyde | 98 | 176–178 |
| 14 | 1-naphthaldehyde | 98 | 175 |
| 15 | biphenyl-4-carbaldehyde | 90 | 238–240 |
| 16 | 4-(dimethylamino)-cinnammaldehyde | 98 | 252–253 |
| 17 | 4-(12-bromododecyloxy) benzaldehyde | 60 | 104–106 |
