Fig. 3. Potential actionable pathways in cancer cells to increase immune checkpoints blockade efficiency.
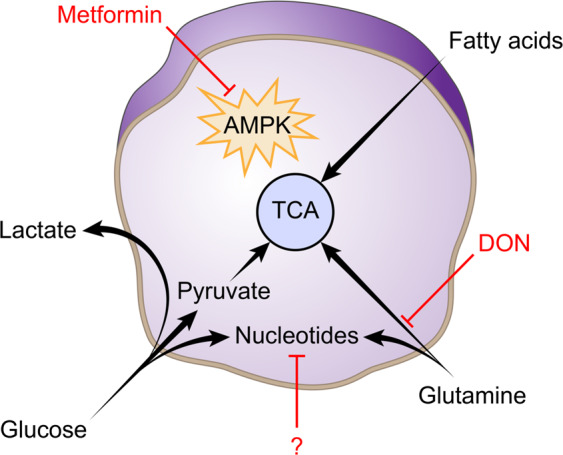
Highly resources demand cancer cells necessary to sustain their proliferation opens several therapeutics opportunities to improve immune checkpoints blockade efficiency. Targeting AMPK especially with metformin of other biguanide derivatives could be an interesting combinatory strategy to increase immunotherapies efficiency. However, regarding the contradictory effects report on PD-L1 expression and degradation, additional exploration needs to fully evaluate the potential of this combination and especially with which immunotherapy (anti-PD1/PD-L1 or anti-CTLA4) targeting AMPK could be a therapeutic option. Glutamine dependency is share between a cancer cell and CD8 effector T cells highlighting glutaminolysis as a potential therapeutic target. Even if using single enzyme inhibitors have shown a modest therapeutic effect, recent results obtained with DON, a glutamine analog that inhibits a large spectrum of glutaminolysis enzyme have shown a spectacular effect by inducing a glutaminolysis that cannot be compensated by cancer cells contrariwise of CD8 T cells that increase glucose uptake to fuel PPP activity and maintain their anti-tumor activity. Nucleotides metabolism emerges as a promising therapeutic option especially with recent studies implicating nucleotide imbalance in the generation of a specific mutation pattern that predicts response to immunotherapy. However, exploration is needed to explore the consequences of nucleotides metabolism intervention, especially in the tumor stroma.
