Abstract
To investigative whether radiomics features in bilateral hippocampi from MRI can identify temporal lobe epilepsy (TLE). A total of 131 subjects with MRI (66 TLE patients [35 right and 31 left TLE] and 65 healthy controls [HC]) were allocated to training (n = 90) and test (n = 41) sets. Radiomics features (n = 186) from the bilateral hippocampi were extracted from T1-weighted images. After feature selection, machine learning models were trained. The performance of the classifier was validated in the test set to differentiate TLE from HC and ipsilateral TLE from HC. Identical processes were performed to differentiate right TLE from HC (training set, n = 69; test set; n = 31) and left TLE from HC (training set, n = 66; test set, n = 30). The best-performing model for identifying TLE showed an AUC, accuracy, sensitivity, and specificity of 0.848, 84.8%, 76.2%, and 75.0% in the test set, respectively. The best-performing radiomics models for identifying right TLE and left TLE subgroups showed AUCs of 0.845 and 0.840 in the test set, respectively. In addition, multiple radiomics features significantly correlated with neuropsychological test scores (false discovery rate-corrected p-values < 0.05). The radiomics model from hippocampus can be a potential biomarker for identifying TLE.
Subject terms: Neuroscience, Biomarkers, Medical research, Neurology
Introduction
Temporal lobe epilepsy (TLE) is the most frequent type of focal epilepsy, and also the type which is most refractory to drug treatment1. For many of these patients, surgical resection of the epileptic focus offers a viable treatment option to eliminate seizures, and noninvasive imaging plays a pivotal role in precisely identifying the epileptogenic zone2,3. One of the histopathologic landmarks of TLE is hippocampal sclerosis, which presents in approximately 54% of surgically treated drug-refractory patients4,5. Previous neuroimaging studies on TLE have mainly focused on volumetric analysis, diffusion tensor image (DTI) studies, or functional MRI studies6–8. Volumetric analyses revealed abnormalities in the hippocampus ipsilateral to the seizure focus, which is the hallmark imaging finding in TLE9–11. However, these studies focused on single parameters such as mean apparent diffusion coefficient, mean fractional anisotropy, or volume rather than focusing on the spatial distribution of heterogeneity. Also, it is known that TLE patients show cognitive impairments, affecting not only memory, but also a broad array of cognitive capacities including executive functions, language, and sensorimotor skills12–14. Previous MRI studies have shown structural and functional damage associated with cognitive impairments in TLE patients12,15–17, but these studies have also focused on single conventional parameters.
Radiomics is a rapidly developing study field that extracts comprehensive and automated quantifications of radiographic phenotypes using data characterization algorithms18. Because radiomics models use high-throughput imaging features, they are able to discover hidden information that is inaccessible when using single-parameter approaches. High-dimensional radiomics data provide insight into intraregional heterogeneity and reflects the spatial complexity of a disease, which are invisible to the human eye. Compared with previous techniques that process medical images for visual examination, radiomics introduces a new method for data mining and thus offers novel opportunity for clinically assisted diagnosis19. We hypothesized that radiomics features from routine conventional MRI would result in distinct combinations of imaging parameters that identify TLE. We additionally assessed whether these radiomics features were correlated with cognitive impairments in these TLE patients.
Methods
Patient population
Written informed consent was obtained from legally authorized representatives of TLE patients and HCs, and the study protocol was approved by the local Ethical Committee at Ewha Medical Center. All the experiment protocol for involving humans was in accordance to guidelines of national/international/institutional or Declaration of Helsinki. Sixty-six TLE patients who had visited Ewha Womans University Mokdong Hospital for both long-term video encephalographic (EEG) monitoring and MRI scans between July 2013 and August 2018 were enrolled. The clinical diagnosis of TLE was made according to the criteria of the International League Against Epilepsy20,21. Based on electroclinical findings, we included TLE patients with recurrent unprovoked seizures, with or without secondary generalization, originating from the temporal lobe.
All patients had unilateral temporal interictal spikes or unilateral temporal lobe seizure onset on EEG. TLE patients were further divided into those with a right-sided (n = 35) or left-sided (n = 31) seizure focus. The seizure focus was determined by predominantly ipsilateral interictal epileptic abnormalities (70% cutoff) and unequivocal seizure onset recorded during prolonged video-EEG monitoring in all patients22. Patients were excluded from the study if they had contralateral or extratemporal epileptiform discharges on EEG, previous brain surgery, chronic medical illness with central nervous system involvement other than epilepsy, contraindication for MRI, or history of drug abuse or psychiatric illness other than axis I depressive disorders. For the control group, 65 healthy controls (HCs) (age = 40.5 ± 12.1 years, 30 females) were recruited. The HCs had no history of neurological disorders and no MRI abnormalities. An institutional cohort of 131 subjects (66 TLE patients and 65 HCs) was enrolled in the study.
To differentiate TLE from HCs, the institutional cohort was split into training (n = 90) and test (n = 41) sets, with stratification for TLE presence. Also, because TLE is a lateralized disease, we performed subgroup analyses in (1) right TLE vs. HC, in which right TLE and HC were included and semi-randomly allocated to training (n = 69) and test (n = 31) sets, and (2) left TLE vs. HC, in which left TLE and HC were included and semi-randomly allocated to training (n = 66) and test (n = 30) sets.
Verbal memory function, visual memory function, language function, and frontal executive function were assessed using a Korean version of the California Verbal Learning Test (CVLT)23, Rey Complex Figure Test (RCFT)24, Korean version of the Boston Naming Test (K-BNT)25, sematic Controlled Oral Word Association Test (COWAT) and Stroop test, respectively (detailed information in Supplementary Material S1). The Mini-Mental State Examination (MMSE) was performed in TLE patients only.
MRI protocols
MRI was performed using a 3.0 T scanner (Philips Achieva v 2.6, Philips, Andover, MA, USA) in all subjects. The MRI included T1-weighted (TR/TE 1160/4.19 ms; field of view 140 × 250 mm, section thickness 1.2 mm, matrix 256 × 192 mm, flip angle 15°), T2-weighted axial/oblique coronal and FLAIR axial/oblique coronal images (5 mm section thickness for each sequence), as well as axial T2* GRE (TR/TE 720/16 ms; field of view 220 × 220 mm, section thickness 5 mm, matrix 256 × 229 mm, flip angle 18°) in TLE patients. HCs underwent identical T1-weighted MRI sequences.
Image preprocessing and radiomics feature extraction
Preprocessing of the images was performed to standardize the data analysis across patients. We resampled the original images to a 1 mm isovoxel and used FreeSurfer 5.3.0 (surfer.nmr.mgh.harvard.edu) to obtain subject-specific masks of brain regions as defined by the Desikan-Killany atlas26. This procedure involved motion correction of T1-weighted images, removal of non-brain tissue27, automatic Talairach transformation, segmentation of subcortical white matter and deep gray matter structures28, intensity normalization, tessellation of the gray matter/white matter boundary29, automated topology correction, and surface deformation following intensity gradients30. Once the cortical models were complete, they were registered to a spherical atlas, and the cerebral cortex was parcellated into units with respect to gyral sand sulcal structures31. We mapped the brain parcellation mask for each subject from FreeSurfer space to the isovoxel native space and extracted two regions of interest (ROI) masks (right and left hippocampi). We visually checked for segmentation or registration errors by overlaying each subject’s native-space-transformed ROI masks onto their T1-weighted images.
Non-uniform low-frequency intensity was removed by applying the N4 bias correction algorithm. Next, arbitrary T1 signal intensities were normalized using the Whitestripe normalization algorithm32. All images were resampled to 1 mm isovoxels across all patients.
The following radiomics features were extracted from each ROI on T1-weighted images using an open-source package (PyRadiomics, version 1.3.0)33: (1) 14 shape features, (2) 18 first-order features, and (3) 61 s-order features (including gray level co-occurrence matrix, gray level run-length matrix, gray-level size zone matrix, and neighboring gray tone difference matrix) (detailed information on Supplementary Table S1). A total of 186 radiomics features were extracted (93 features x two ROIs [right and left hippocampus]).
Statistical analysis
For univariate analysis of baseline characteristics and neuropsychological test scores, either the Student’s t-test or Mann Whitney’s U test was used for continuous variables, depending on the normality of the data. Chi-square test was performed for categorical variables. Statistical analysis was performed using the statistical software R (version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria).
Radiomics feature selection and machine learning models
All imaging features were normalized using z-score normalization. For feature selection, the F-score, least absolute shrinkage and selection operator (LASSO), and mutual information (MI) with ten-fold cross validation was applied. After feature selection, the radiomics classifiers were constructed by machine learning models including support vector machine (SVM), logistic regression (LR), or AdaBoost, with ten-fold cross-validation.
Hyperparameters were optimized by random search. Thus, a total of 9 combinations of machine learning algorithms were trained and validated. Performance was evaluated in the training sets and validated in the test sets to differentiate the entire TLE group from HC group, without controlling the alignment of the epileptic side. Further analysis was performed to differentiate the ipsilateral TLE group from the HC group. In subgroup analyses of (1) right TLE vs. HC and (2) left TLE vs. HC, feature selection and machine learning were performed separately. In addition, to overcome data imbalances in subgroup analyses, each machine learning model was trained (1) without over-sampling, or (2) with SMOTE (synthetic minority over-sampling technique)34,35. Thus, a total of 18 combinations of machine learning algorithms and over-sampling were trained and validated for each subgroup. Performance was evaluated in the training set and validated in the test set. Area under the curve (AUC), accuracy, sensitivity, and specificity were estimated.
Based on the radiomics classification model in the training set, the best combination of feature selection, classification methods, and subsampling in each model was used in the test set.
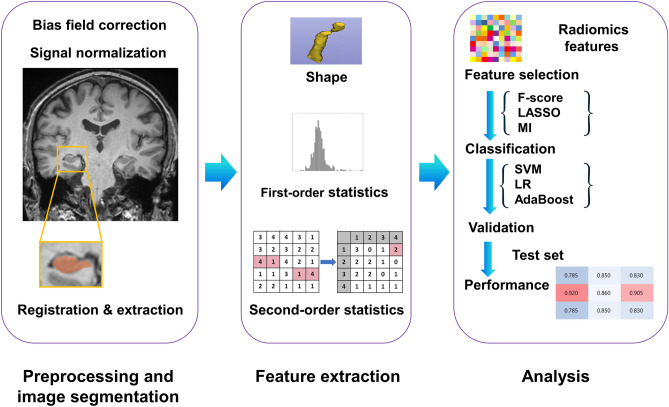
The different feature selection, over-sampling, and classification methods computed using Machine learning were performed using Python 3 with Scikit-Learn library v 0.21.2. The threshold for statistical significance was set at p < 0.05. The overall process is shown in Fig. 1.
Figure 1.
Workflow of image processing, radiomics feature extraction, and machine learning.
Comparison of the diagnostic performance with that of human readers
We tested the diagnostic performance to differentiate TLE from HC in training and test set on T1-weighted images by a consensus of 2 readers (a neuroradiologist with 9 years of experience and a neurologist with 27 years of experience, respectively), who were blinded to the clinical information. The performance of radiomics model and human readers were compared using AUC with DeLong’s method36.
Correlations between radiomics features and neurocognitive function
Pearson’s correlation coefficient was calculated to evaluate the relationships between the identified radiomics features and significantly different neuropsychological results between TLE and HCs (FDR-corrected p < 0.05).
Results
Subject characteristics
The baseline characteristics and neuropsychological test results of TLE patients and HCs are summarized in Supplementary Table S2. TLE patients showed significant cognitive decrements in all cognitive domains (including language, verbal memory, visual memory, and frontal/executive function), compared with HCs. Left TLE patients showed worse language functions than right TLE patients on K-BNT scores (32.9 vs. 58.0, p = 0.002). Otherwise, there were no significant differences in verbal memory, visual memory, and frontal/executive functions between right TLE and left TLE patients (Supplementary Table S3).
The clinical characteristics of the subjects in the training set and test set are summarized in Table 1. There were no differences in the clinical characteristics between the training and test sets.
Table 1.
Clinical characteristics in the training and test sets.
| Training set (n = 90) | Test set (n = 41) | p value* | |
|---|---|---|---|
| TLE vs HC | |||
| Age (years) | 41.8 ± 11.4 | 40.8 ± 13.6 | 0.668 |
| Sex (female) | 46 (51.1) | 23 (56.1) | 0.596 |
| Subjects no. (%) | 0.989 | ||
| HC | 45 (50) | 20 (48.8) | |
| Right TLE | 24 (26.7) | 11 (26.8) | |
| Left TLE | 21 (23.3) | 10 (24.4) | |
| Training set (n = 69) | Test set (n = 31) | p value* | |
|---|---|---|---|
| Right TLE vs HC | |||
| Age (years) | 41.0 ± 11.6 | 42.5 ± 12.8 | 0.587 |
| Sex (female) | 33 (47.8) | 18 (58.1) | 0.344 |
| Subjects no. (%) | 0.946 | ||
| HC | 45 (65.2) | 20 (64.5) | |
| Right TLE | 24 (34.8) | 11 (35.5) | |
| Training set (n = 66) | Test set (n = 30) | p value* | |
|---|---|---|---|
| Left TLE vs HC | |||
| Age (years) | 41.6 ± 11.8 | 39.3 ± 13.3 | 0.393 |
| Sex (female) | 31 (47) | 17 (56.7) | 0.378 |
| Subjects no. (%) | 0.883 | ||
| HC | 45 (68.2) | 20 (66.7) | |
| Left TLE | 21 (31.8) | 10 (33.3) | |
Data are number of subjects. Numbers in parentheses are percentages.
HC healthy control, TLE temporal lobe epilepsy.
*p values were calculated using Student’s t-test for continuous variables and Chi-square test for categorical variables, to compare subject characteristics of the training and test set.
Radiomics features and classification performance
Entire TLE vs. HC
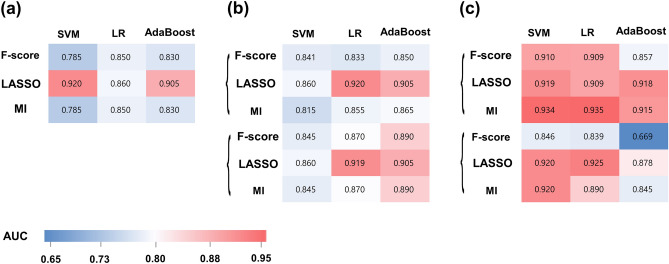
Table 2 summarizes the results of the best performing models in the training and test sets. The performance of the 9 combination of models in the training set is shown in Fig. 2a. In the training set, the AUCs of the models ranged from 0.680 to 0.920. LASSO feature selection and SVM showed the best diagnostic performance in the training set, with an AUC, accuracy, sensitivity, and specificity of 0.920 (95% confidence interval [CI] 0.870–0.970), 80.5%, 85.7%, and 80%, respectively. The selected features (10 from right and 6 from left hippocampus) consisted of 2 first-order features, 10 s-order features, and 4 shape features (detailed information in Supplementary Table S4). In the test set, the LASSO feature selection with SVM showed an AUC, accuracy, sensitivity, and specificity of 0.848 (95% CI 0.731–0.964), 84.8%, 76.2%, and 75%, respectively.
Table 2.
Diagnostic performance of the best performing machine learning model in the training set and the test set.
| Feature selection | No. of features | Classification + subsampling | Training set | Test set | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | Accuracy (%) | Sensitivity (%) | Specificity (%) | AUC (95% CI) | Accuracy (%) | Sensitivity (%) | Specificity (%) | |||
| TLE vs HC | ||||||||||
| LASSO | 16 | SVM + none | 0.920 (0.870–0.970) | 80.5 | 85.7 | 80 | 0.848 (0.731–0.964) | 84.8 | 76.2 | 75 |
| Right TLE vs HC | ||||||||||
| F-score | 30 | LR + SMOTE | 0.920 (0.870–0.970) | 81.1 | 94 | 68.5 | 0.845 (0.723–0.968) | 77.4 | 72.7 | 80 |
| Left TLE vs HC | ||||||||||
| LASSO | 18 | LR + none | 0.935 (0.893–0.977) | 87.8 | 82.5 | 93 | 0.840 (0.699–0.981) | 73.3 | 70 | 75 |
AUC area under the curve, CI confidence interval, HC healthy control, LASSO least absolute shrinkage and selection operator, LR logistic regression, MI mutual information, SMOTE synthetic minority over-sampling technique, SVM support vector machine, TLE temporal lobe epilepsy.
Figure 2.
Heatmap of AUC values achieved from the machine learning classifiers in the training sets for (a) differentiating TLE from HC, (b) differentiating right TLE from HC, and (c) differentiating left TLE from HC. AUC area under the curve, MI mutual information, LASSO least absolute shrinkage and selection operator, LR logistic regression, MI mutual information, SMOTE synthetic minority over-sampling technique, SVM support vector machine.
Right TLE vs. HC
The performance of the 18 combination of models in the training set is shown in Fig. 2b. In the training set, the AUCs of the models ranged from 0.734 to 0.891. F-score feature selection, with LR and SMOTE showed the best diagnostic performance in the training set, with an AUC, accuracy, sensitivity, and specificity of 0.920 (95% CI 0.870–0.970), 81.1%, 95%, and 68.5% in the test set, respectively.
The selected features (14 from right and 16 from left hippocampus) included 23 first-order features, 3 s-order features, and 4 shape features. In the test set, the F-score feature selection with LR and SMOTE showed an AUC, accuracy, sensitivity, and specificity of 0.845 (95% CI 0.723–0.968), 77.4%, 72.7%, and 80%, respectively.
Left TLE vs. HC
The performance of the 18 combination of models in the training set is shown in Fig. 2c. In the training set, the AUCs of the models ranged from 0.845 to 0.935. LASSO feature selection, LR, with SMOTE showed the best diagnostic performance in the training set, with an AUC, accuracy, sensitivity, and specificity of 0.935 (95% CI 0.893–0.977), 87.8%, 982.5%, and 93% in the test set, respectively.
The selected features (5 from right and 13 from left hippocampus) included 2 first-order features, 12 s-order features, and 5 shape features. In the test set, the LASSO feature selection with SVM showed an AUC, accuracy, sensitivity, and specificity of 0.840 (95% CI 0.699–0.981), 73.3%, 70%, and 75%, respectively.
Comparison of the diagnostic performance of human readers
The reviewers correctly identified 14 and 7 cases of TLE in the training and test sets. The AUC, accuracy, sensitivity, and specificity was 0.617 (0.452–0.764), 17.1%, 23.3%, and 100% in the test set, respectively. The radiomics model showed significantly better performance than human readers did (p-value = 0.001).
Correlations between radiomics features and neuropsychological scores
Among the 16 significant radiomics features from LASSO in differentiating TLE from HC, three were significantly correlated with K-BNT score, two were significantly correlated with CVLT direct recall score, and one was significantly correlated with RCFT immediate recall score (all FDR-corrected p-values < 0.05) (Table 3).
Table 3.
Summary of radiomics features showing significant correlation with neuropsychological test results.
| Radiomics features | Pearson’s correlation coefficients | FDR-corrected p-value |
|---|---|---|
| K-BNT | ||
| right_T1_GLSZM_SmallAreaLowGrayLevelEmphasis | − 0.303 | 0.022 |
| Left_T1_shape_MeshVolume | 0.378 | 0.004 |
| Left_T1_shape_Maximum2DDiameterColumn | 0.296 | 0.023 |
| CVLT-direct recall | ||
| Left_T1_shape_Maximum2DDiameterRow | 0.230 | 0.003 |
| Left_T1_shape_MeshVolume | 0.241 | 0.016 |
| RCFT-direct recall | ||
| Left_T1_shape_MeshVolume | 0.308 | 0.020 |
CVLT California verbal learning test, FDR false discovery rate, GLSZM gray level size zone matrix, K-BNT Korean version of the Boston Naming Test, RCFT Rey complex figure test.
Discussion
In our study, radiomics analysis of hippocampus showed promising results, with an AUC of 0.848, 0.845, and 0.840 for identifying the entire TLE as well as right TLE and left TLE in the test set, respectively. Surgical resection of the epileptogenic zone, the region which is necessary and sufficient for the initiation of seizures, is an additional treatment option which can achieve seizure freedom37. Our radiomics approach with machine learning may be applicable to surgical planning by early identification, and thus allow for an improved patient selection and counseling. Our radiomics model was particularly useful in showing better diagnostic performance than visual inspection of human readers, which is the current standard approach, showing its utility. Previous radiomics studies in the neuroradiology field have mostly focused on brain tumors38–42. Our study shows that a radiomics model can be a useful biomarker for identifying TLE.
Notably, our model included both right and left TLE in the entire TLE dataset. TLE is considered to display a strong asymmetrical distribution of abnormalities such as volume loss or white matter abnormalities, primarily observed ipsilateral to the seizure onset site7. However, it is also known that the contralateral side of seizure onset may also show volume loss or white matter alterations, although less prominently than the ipsilateral side7,17,43,44. Our radiomics model showed a good performance (AUC 0.848) in differentiating the entire TLE from HC, indicating that radiomics has the potential for creating a generalized model that is not influenced by the laterality of TLE. Our results are in agreement with previous studies reporting that microstructural changes precede macroscopic atrophy45, and that radiomics may reflect microstructural information different from that provided by volumetric measures.
Neuropathological research has revealed various patterns of neuronal cell loss or gliotic changes within the hippocampus, including hippocampal sclerosis, which is the most common histopathologic abnormality46,47. MRI T1 relaxation time is a direct reflection of tissue characteristics and has been reported to independently predict histological measures of neuronal density in TLE48. Such variations in relaxation time, which directly cause variations in MRI signal intensity, may provide information beyond that provided by volumetric measures. Radiomics features, especially second-order features, capture the spatial variation in T1 signal intensity that may reflect the underlying pathophysiology, which may explain our observation.
In our study, we have applied domain knowledge in performing radiomics analysis in bilateral hippocampi, which is the key structure involved in TLE7. However, because radiomics features tend to extract agnostic information that is invisible to human eyes, one cannot assume which feature will be most important in identifying TLE. Thus, we have further narrowed down the significant features by using various feature selection methods. This methodology results in creating a more generalized and stable classifier that is robust against the idiosyncrasies of the training data49,50.
Further, it is known that TLE patients show cognitive impairments, affecting not only memory, but also a broad array of cognitive capacities including executive functions, language and sensorimotor skills12–14. Previous MRI studies have shown structural and functional compromises associated with cognitive impairment in TLE patients12,15–17, but these studies were also focused on single conventional parameters. Other studies have shown dysfunctional networks related to cognitive impairment, seizure severity, or seizure related change in TLE patients in task-based and/or resting-state functional MRI studies8,50–55. In our study, significant correlations were found between radiomics features and neuropsychological test scores, suggesting that radiomics features can also serve as imaging biomarkers for cognitive capacity in TLE patients.
Our study has several limitations. First, this is a retrospective study in a single institution with a relatively small sample size. Further studies with a larger dataset and external validation are warranted for better assessment. Second, because only the hippocampus masks were included, other important regions such as the amygdala and parahippocampus should be investigated in future studies. Third, T2-weighted or FLAIR images were not included in this study because the MRI protocol for healthy controls did not include the aforementioned sequences. Further studies including radiomics features from T2-weighted or FLAIR images should be performed. Fourth, we preserved the laterality of TLE rather than flipping (right to left or left to right TLE) to evaluate the radiomics model. However, flipping would have allowed using sampling algorithms sparingly. We have performed this method because previous studies have shown that right and left TLE demonstrate asymmetrical and different qualities of hippocampal injuries53–58.
In conclusion, radiomics model from the hippocampus can be a potential biomarker for identifying TLE.
Supplementary information
Author contributions
Y.W.P. and H.W.L wrote the main manuscript text. Y.W.P. prepared Figs. 1 and 2. Y.S.C., S.E.K., S.-A.K., and H.J.K. collected the data. D.C., H.K, and S.S.A. conducted and supervised the machine learning process. Y.W.P. performed the biostatistics analyses and K.H. supervised the biostatistics analyses. S.-K.L. supervised the manuscript. All authors reviewed the manuscript.
Funding
This research was supported by the Brain Science Research Program, the Convergent Technology R&D Program for Human Augmentation, and the BK21 plus program through the National Research Foundation (NRF) funded by the Ministry of Science, Information and Communication Technologies and Future Planning of Korea (2017R1A2A2A05069647, 2017R1B5A2086553, 2018M3C1B8016147, 2019M3C1B8090803, and 2020R1A2C2013216) to H.W.Lee. This research was also supported by Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education of Korea (2020R1I1A1A01071648) to Y.W.Park.
Data availability
Our anonymized data can be obtained by any qualified investigators for the purposes of replicating procedures and results, after ethics clearance and approval by all authors.
Code availability
The code for machine learning was modified from our previous available radiomics analysis package (https://github.com/ChoiDM/YBIGTA_AI_HeLP).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-76283-z.
References
- 1.Engel J., Jr Mesial temporal lobe epilepsy: what have we learned? Neuroscientist. 2001;7:340–352. doi: 10.1177/107385840100700410. [DOI] [PubMed] [Google Scholar]
- 2.Lowe AJ, et al. Epilepsy surgery for pathologically proven hippocampal sclerosis provides long-term seizure control and improved quality of life. Epilepsia. 2004;45:237–242. doi: 10.1111/j.0013-9580.2004.35903.x. [DOI] [PubMed] [Google Scholar]
- 3.Antel S, et al. Predicting surgical outcome in temporal lobe epilepsy patients using MRI and MRSI. Neurology. 2002;58:1505–1512. doi: 10.1212/WNL.58.10.1505. [DOI] [PubMed] [Google Scholar]
- 4.Mathern, G. W., Adelson, P. D., Cahan, L. D. & Leite, J. P. in Prog Brain Res135, 237–251 (Elsevier, 2002). [DOI] [PubMed]
- 5.Blumcke I, et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N. Engl. J. Med. 2017;377:1648–1656. doi: 10.1056/NEJMoa1703784. [DOI] [PubMed] [Google Scholar]
- 6.Focke NK, et al. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage. 2008;40:728–737. doi: 10.1016/j.neuroimage.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. 2008;49:741–757. doi: 10.1111/j.1528-1167.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- 8.Cataldi M, Avoli M, de Villers-Sidani E. Resting state networks in temporal lobe epilepsy. Epilepsia. 2013;54:2048–2059. doi: 10.1111/epi.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babb TL, et al. Temporal lobe volumetric cell densities in temporal lobe epilepsy. Epilepsia. 1984;25:729–740. doi: 10.1111/j.1528-1157.1984.tb03484.x. [DOI] [PubMed] [Google Scholar]
- 10.Jack CR, Jr, Sharbrough FW, Marsh WR. Use of MR imaging for quantitative evaluation of resection for temporal lobe epilepsy. Radiology. 1988;169:463–468. doi: 10.1148/radiology.169.2.3174994. [DOI] [PubMed] [Google Scholar]
- 11.Cendes F, et al. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology. 1993;43:719–725. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- 12.Riley JD, et al. Altered white matter integrity in temporal lobe epilepsy: association with cognitive and clinical profiles. Epilepsia. 2010;51:536–545. doi: 10.1111/j.1528-1167.2009.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oyegbile T, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62:1736–1742. doi: 10.1212/01.WNL.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- 14.Bell B, Lin JJ, Seidenberg M, Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat. Rev. Neurol. 2011;7:154. doi: 10.1038/nrneurol.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald CR, et al. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widjaja E, et al. Abnormal white matter correlates with neuropsychological impairment in children with localization-related epilepsy. Epilepsia. 2013;54:1065–1073. doi: 10.1111/epi.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braakman HM, et al. Microstructural and functional MRI studies of cognitive impairment in epilepsy. Epilepsia. 2012;53:1690–1699. doi: 10.1111/j.1528-1167.2012.03624.x. [DOI] [PubMed] [Google Scholar]
- 18.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2015;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JE, Kim HS. Radiomics as a quantitative imaging biomarker: practical considerations and the current standpoint in neuro-oncologic studies. Nucl. Med. Mol. Imaging. 2018;52:99–108. doi: 10.1007/s13139-017-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher RS, et al. Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 21.Scheffer IE, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernasconi N, et al. Entorhinal cortex atrophy in epilepsy patients exhibiting normal hippocampal volumes. Neurology. 2001;56:1335–1339. doi: 10.1212/wnl.56.10.1335. [DOI] [PubMed] [Google Scholar]
- 23.Kim JK, Kang Y. Normative study of the Korean-California Verbal Learning Test (K-CVLT) Clin. Neuropsychol. 1999;13:365–369. doi: 10.1076/clin.13.3.365.1740. [DOI] [PubMed] [Google Scholar]
- 24.Shin M-S, Park S-Y, Park S-R, Seol S-H, Kwon JS. Clinical and empirical applications of the Rey-Osterrieth complex figure test. Nat. Protoc. 2006;1:892. doi: 10.1038/nprot.2006.115. [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Na DL. brief report normative data on the Korean version of the Boston naming test. J. Clin. Exp. Neuropsychol. 1999;21:127–133. doi: 10.1076/jcen.21.1.127.942. [DOI] [PubMed] [Google Scholar]
- 26.Desikan RS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Ségonne F, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Fischl B, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- 29.Ségonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans. Med Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 30.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 31.Fischl B, et al. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 32.Shinohara, R. T. et al. Statistical normalization techniques for magnetic resonance imaging. NeuroImage Clin.6, 9–19 (2014). [DOI] [PMC free article] [PubMed]
- 33.van Griethuysen, J. J. et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res.77, e104–e107 (2017). [DOI] [PMC free article] [PubMed]
- 34.Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP. SMOTE: synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002;16:321–357. doi: 10.1613/jair.953. [DOI] [Google Scholar]
- 35.Chawla, N. V. in Data mining and knowledge discovery handbook 875–886 (Springer, 2009).
- 36.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 37.Abbasi B, Goldenholz DM. Machine learning applications in epilepsy. Epilepsia. 2019;60:2037–2047. doi: 10.1111/epi.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park Y, et al. Whole-tumor histogram and texture analyses of DTI for evaluation of IDH1-mutation and 1p/19q-Codeletion Status in World Health Organization Grade II Gliomas. Am. J. Neuroradiol. 2018;39:693–698. doi: 10.3174/ajnr.A5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park, Y. W. et al. Radiomics and machine learning may accurately predict the grade and histological subtype in meningiomas using conventional and diffusion tensor imaging. Eur. Radiol. 1–9 (2018). [DOI] [PubMed]
- 40.Park YW, et al. Radiomics MRI phenotyping with machine learning to predict the grade of lower-grade gliomas: a study focused on nonenhancing tumors. Korean J. Radiol. 2019;20:1381. doi: 10.3348/kjr.2018.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bae S, et al. Radiomic MRI phenotyping of glioblastoma: improving survival prediction. Radiology. 2018;289:797–806. doi: 10.1148/radiol.2018180200. [DOI] [PubMed] [Google Scholar]
- 42.Park CJ, et al. Diffusion tensor imaging radiomics in lower-grade glioma: improving subtyping of isocitrate dehydrogenase mutation status. Neuroradiology. 2019;62:319–326. doi: 10.1007/s00234-019-02312-y. [DOI] [PubMed] [Google Scholar]
- 43.Otte WM, et al. A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia. 2012;53:659–667. doi: 10.1111/j.1528-1167.2012.03426.x. [DOI] [PubMed] [Google Scholar]
- 44.Bonilha L, et al. Texture analysis of hippocampal sclerosis. Epilepsia. 2003;44:1546–1550. doi: 10.1111/j.0013-9580.2003.27103.x. [DOI] [PubMed] [Google Scholar]
- 45.Weston PS, Simpson IJ, Ryan NS, Ourselin S, Fox NC. Diffusion imaging changes in grey matter in Alzheimer’s disease: a potential marker of early neurodegeneration. Alzheimers Res. Ther. 2015;7:47. doi: 10.1186/s13195-015-0132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blümcke I, et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE commission on diagnostic methods. Epilepsia. 2013;54:1315–1329. doi: 10.1111/epi.12220. [DOI] [PubMed] [Google Scholar]
- 47.Muhlhofer W, Tan YL, Mueller SG, Knowlton R. MRI-negative temporal lobe epilepsy—what do we know? Epilepsia. 2017;58:727–742. doi: 10.1111/epi.13699. [DOI] [PubMed] [Google Scholar]
- 48.Goubran M, et al. Magnetic resonance imaging and histology correlation in the neocortex in temporal lobe epilepsy. Ann. Neurol. 2015;77:237–250. doi: 10.1002/ana.24318. [DOI] [PubMed] [Google Scholar]
- 49.Park JE, Park SY, Kim HJ, Kim HS. Reproducibility and generalizability in radiomics modeling: possible strategies in radiologic and statistical perspectives. Korean J. Radiol. 2019;20:1124–1137. doi: 10.3348/kjr.2018.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.An, S., Kang, C. & Lee, H. W. Artificial intelligence and computational approaches for epilepsy. J. Epilepsy Res. 10, 8–17 (2020). [DOI] [PMC free article] [PubMed]
- 51.Rosazza C, et al. Preoperative language lateralization in temporal lobe epilepsy (TLE) predicts peri-ictal, pre-and post-operative language performance: an fMRI study. NeuroImage Clin. 2013;3:73–83. doi: 10.1016/j.nicl.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haneef Z, et al. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia. 2014;55:137–145. doi: 10.1111/epi.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park C, et al. Seizure control and memory impairment are related to disrupted brain functional integration in temporal lobe epilepsy. J. Neuropsychiatry Clin. Neurosci. 2017;29:343–350. doi: 10.1176/appi.neuropsych.16100216. [DOI] [PubMed] [Google Scholar]
- 54.Park C, et al. Interactive effects of seizure frequency and lateralization on intratemporal effective connectivity in temporal lobe epilepsy. Epilepsia. 2018;59:215–225. doi: 10.1111/epi.13951. [DOI] [PubMed] [Google Scholar]
- 55.Lee HW, et al. Altered functional connectivity in seizure onset zones revealed by fMRI intrinsic connectivity. Neurology. 2014;83:2269–2277. doi: 10.1212/WNL.0000000000001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmadi ME, et al. Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. Am. J. Neuroradiol. 2009;30:1740–1747. doi: 10.3174/ajnr.A1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernasconi N, et al. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126:462–469. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- 58.Morgan VL, Sonmezturk HH, Gore JC, Abou-Khalil B. Lateralization of temporal lobe epilepsy using resting functional magnetic resonance imaging connectivity of hippocampal networks. Epilepsia. 2012;53:1628–1635. doi: 10.1111/j.1528-1167.2012.03590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our anonymized data can be obtained by any qualified investigators for the purposes of replicating procedures and results, after ethics clearance and approval by all authors.
The code for machine learning was modified from our previous available radiomics analysis package (https://github.com/ChoiDM/YBIGTA_AI_HeLP).