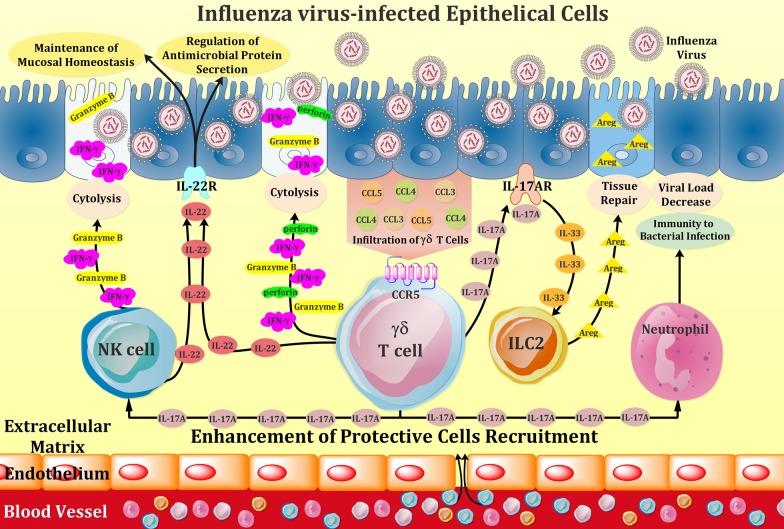
Fig. 1.
Schematic representation of effector mechanisms of γδ T cells in response to influenza virus infection of respiratory epithelium. Upon infection with influenza virus, infected cells secrete CCL3, CCL4, and CCL5 chemokines and thereby recruit γδ T cells, harnessing chemokine-binding CCR5 receptor, to the site of infection. Activated effector γδ T cells release cytokines and chemokines such as IFN-γ, IL-17, IL-22, as well as cytolytic proteins including perforin and granzyme B, which can directly lyse the infected cells and can also recruit other immune cells such as natural killer (NK) cells and neutrophils to aid killing or healing infected cells. Moreover, γδ T cells expressing IL-17A, binds to IL-17A receptor on lung epithelial cells, producing IL-33 and thereafter drive colonic group 2 innate lymphoid cell (ILC2) activation during influenza virus infection. The activated ILC2 produces Amphiregulin (Areg), which participates in pulmonary tissue repair upon influenza infection. In addition, IL-22, released from both γδ T cells and activated NK cells, is effective in preserving the homeostasis of mucosal barrier of influenza-infected respiratory tract and also plays a key regulatory role in microbial host defense after influenza infection