Figure 3.
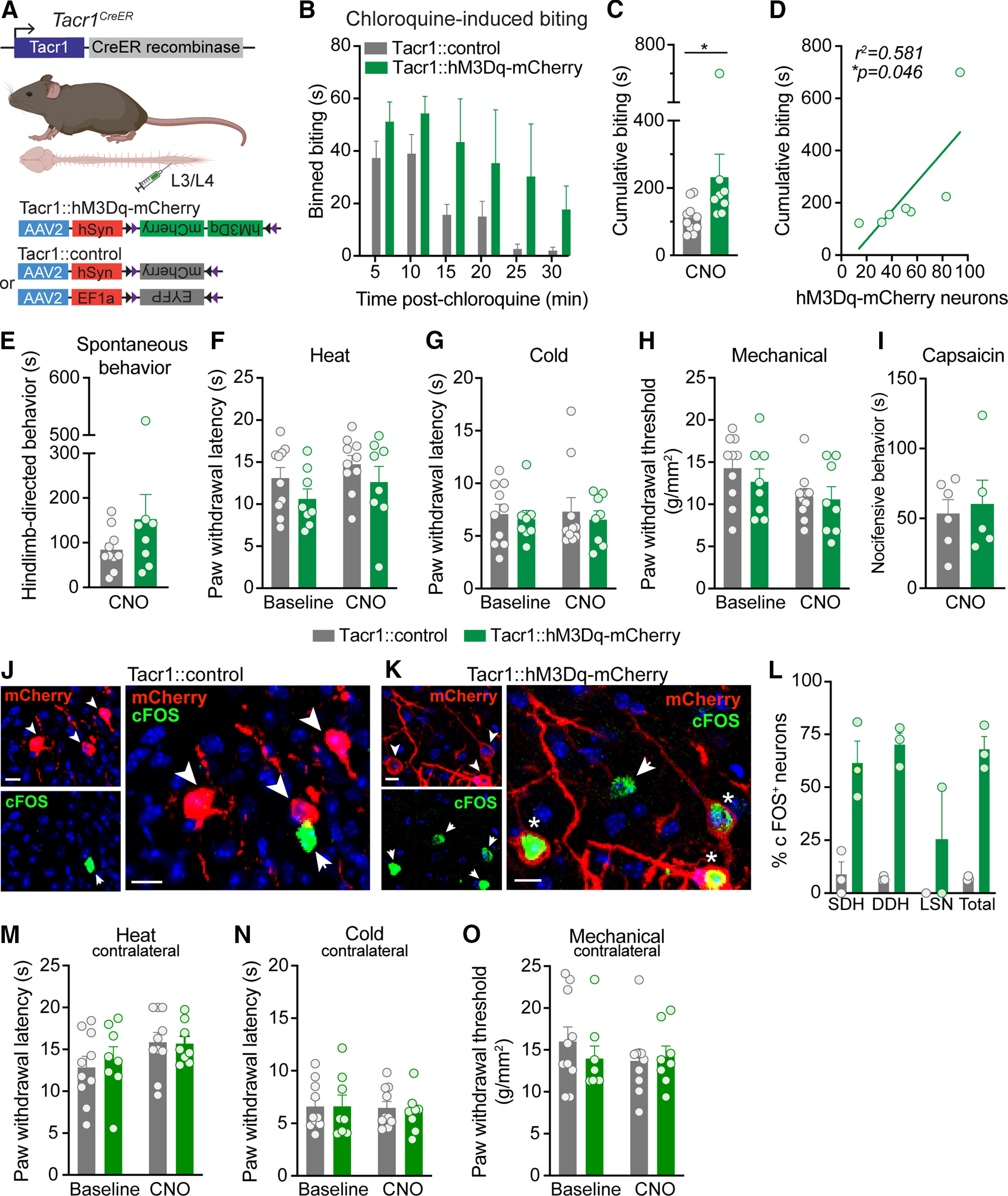
Chemogenetic activation of Tacr1CreER spinal neurons preferentially increases itch. A, Strategy for selectively targeting Tacr1CreER spinal neurons. To gain chemogenetic access to Tacr1CreER spinal neurons, unilateral intraspinal viral injections of AAV2-hSyn-DIO-hM3Dq-mCherry (Tacr1::hM3Dq-mCherry) and either AAV2-hSyn-DIO-mCherry or AAV2-EF1a-DIO-EYFP (Tacr1::control) were targeted to the lumbar dorsal horn of Tacr1CreER mice. B, Duration of site-directed biting in response to an intradermal chloroquine injection (100 μg/5 μl) into the calf following systemic CNO administration (5 mg/kg, i.p.) in Tacr1::control and Tacr1::hM3Dq-mCherry mice, binned over time (n = 8–10 mice/group). C, CNO administration significantly increased the cumulative duration of calf biting of Tacr1::hM3Dq-mCherry mice compared with Tacr1::controls (Mann–Whitney U test, *p = 0.0155, U = 13, df = 16, n = 8–10 mice/group). D, The duration of cumulative chloroquine-induced biting in Tacr1::hM3Dq-mCherry mice was significantly correlated with the number of recombined hM3Dq-mCherry neurons (Pearson's correlation, *p = 0.0463, r2 = 0.581, r = 0.762, n = 7 mice). E, Chemogenetic activation of Tacr1CreER neurons with CNO did not affect pain or itch hindlimb-directed spontaneous behaviors of Tacr1::hM3Dq-mCherry mice compared with Tacr1::controls (Mann–Whitney U test, p = 0.315, U = 28, df = 16, n = 8–10 mice/group). Hindpaw nociceptive withdrawal thresholds to (F) heat (two-way RM ANOVA, time × virus, p = 0.889, F(1,16) = 0.0202, n = 8–10 mice/group), (G) cold (two-way RM ANOVA, time × virus, p = 0.886, F(1,16) = 0.0213, n = 8–10 mice/group), and (H) mechanical (two-way RM ANOVA, time × virus, p = 0.520, F(1,16) = 0.432, n = 8–10 mice/group) stimuli were unchanged following CNO administration in Tacr1::hM3Dq-mCherry mice compared with Tacr1::controls. Across all nociceptive withdrawal tests, there was no main effect of virus (heat: p = 0.124, F = 2.63, cold: p = 0.592, F = 0.299, mechanical: p = 0.535, F = 0.402), indicating that hM3Dq-mCherry expression alone did not affect baseline nociceptive withdrawal thresholds compared with expression of control virus. I, CNO administration did not alter the duration of nocifensive behaviors in response to intraplantar capsaicin (0.001% capsaicin, 10 μl) in Tacr1::hM3Dq-mCherry mice relative to Tacr1::controls (Student's t test, p = 0.729, t = 0.358, df = 9, n = 5–6 mice/group). Representative IHC images of lumbar spinal cord sections demonstrating mCherry (red) and cFOS (green) expression in (J) Tacr1::control and (K) Tacr1::hM3Dq-mCherry mice 90 min following CNO administration, coinciding with the timeframe of behavioral testing. Scale bars, 10 μm. Arrowhead or arrow, labeled neuron; Asterisk, dual-labeled neuron. L, Quantification of cFOS+ mCherry neurons shows that CNO induced significantly more cFOS expression in Tacr1::hM3Dq-mCherry neurons compared with Tacr1::neurons (mixed effects models, virus × spatial distribution, **p = 0.0072, n = 3 mice/group). Chemogenetic activation of Tacr1CreER neurons with CNO administration did not affect the contralateral hindpaw withdrawal thresholds of Tacr1::hM3Dq-mCherry mice to (M) heat (two-way RM ANOVA, time × virus, p = 0.699, F(1,16) = 0.155, n = 8–10 mice/group), (N) cold (two-way RM ANOVA, time × virus, p = 0.753, F(1,16) = 0.102, n = 8–10 mice/group), or (O) mechanical (two-way RM ANOVA, time × virus, p = 0.0.338, F(1,16) = 0.789, n = 8–10 mice/group) stimuli relative to Tacr1::controls. B, C, E–I, L–O, Data are shown as mean ± SEM, with open circles representing individual mice.
