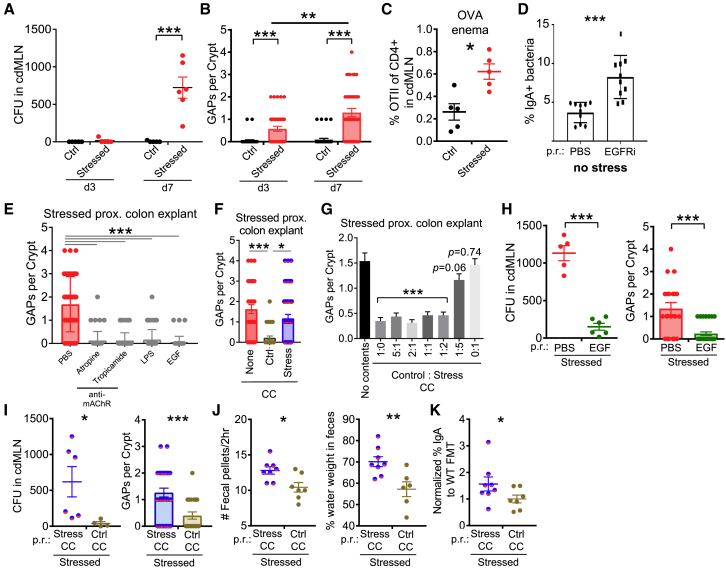
Figure 2.
Stress-Induced Dysbiosis Leads to GAP-Mediated Bacterial Translocation and Diarrheal Signs
(A) Stress-induced bacterial translocation. Aerobic colony-forming units (CFUs) were assessed in the cdMLN on the indicated days 1 h after stress (n = 4–6).
(B) Stress-induced GAPs. Number of open GAPs per crypt from the proximal colon of stressed mice on the indicated days were assessed by rhodamine-dextran staining (n = 4–6).
(C) Presentation of GAP-dependent luminal antigens to T cells. Naive congenically marked OTII cells (105) were transferred on day 6 of stress, followed by 25 mg ovalbumin p.r. on day 7, and analysis 3 days later for the percentage of OTII cells of all CD4 T cells in cdMLN (n = 5).
(D) Increased IgA+ fecal bacteria in non-stressed mice with open GAPs. GAP formation induced via intraperitoneal EGFRi injection for 7 days and IgA+ fecal bacteria assessed on day 8 (n = 10, 3 experiments).
(E) Closure of GAPs via modulation of ACh/Myd88 pathway. Proximal colon explants, obtained from stressed mice 1 h after the last cycle on day 7, were treated with pan or selective cholinergic antagonists (atropine, tropicamide), LPS, or EGF, and assessed for the number of open GAPs per crypt (n = 4–5).
(F and G) Closure of GAPs ex vivo by control cecal contents (CCs). Colon explants of stressed mice were treated with vehicle, control, or stressed CC (F) or varying ratios of control and stressed CCs (G), and then assessed for GAPs (n = 4–6; Student’s t test comparing different ratios to vehicle treatment).
(H) EGF rescues GAP opening and bacterial translocation during stress. EGF was administered p.r. to mice after 2 h of stress every day, and on day 8 bacterial translocation (CFUs in cdMLN) and open GAPs in proximal colon explants were assessed (n = 5–6, 2 expts).
(I–K) Fecal microbiota transplantation (FMT) partially rescues the effects of stress. Stressed mice were given repeated FMT p.r. of either stressed or control CCs daily after each stress cycle and assessed for the following (n = 6–8): (I) bacterial translocation by CFUs in cdMLN and number of open GAPs in proximal colon assessed 1–2 h after the last day of stress on day 7; (J) diarrhea signs by number of fecal pellets expelled during the 2-h stress cycle (average of days 6 and 7) and percentage of fecal water weight assessed on day 7 before stress; and (K) frequency of IgA+ bacteria normalized to each experiment’s control FMT average assessed on day 7 before stress.
The data are presented as means ± SEMs and Student’s t test. Two to three independent experiments for all of the panels. ∗p < 0.05, ∗∗p < 0.005, and ∗∗∗p < 0.0005.