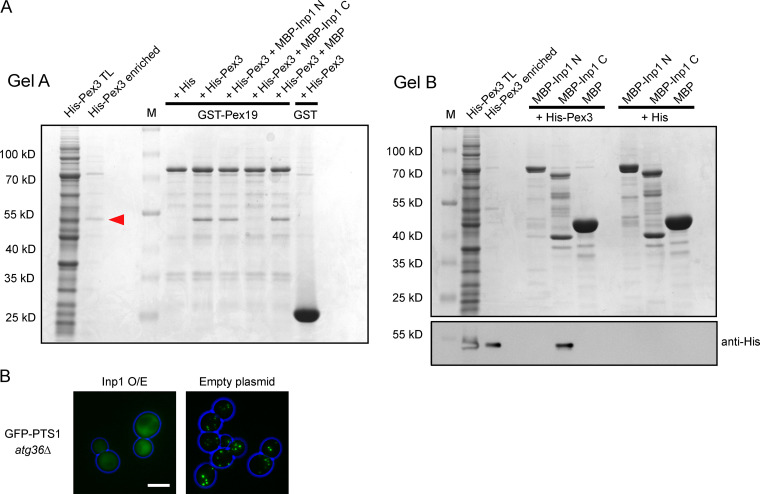
Figure S2.
Inp1 competes with Pex19 in vitro for Pex3 binding. (A) E. coli total lysates expressing His-Pex3 (40–441) or His-tag only were mixed with E. coli total lysates containing MBP-Inp1 N (1–280), MBP-Inp1 C (281–420), or MBP. These lysate mixtures were then incubated with GST-Pex19 or GST and immobilized on glutathione Sepharose beads. After this incubation, the unbound fraction was removed and incubated with amylose beads. The glutathione Sepharose (gel A) and amylose beads (gel B) were washed extensively. Protein was eluted from glutathione Sepharose beads with protein loading buffer, and bound fractions were analyzed by SDS-PAGE and Coomassie staining of the gel (gel A). A lane was included with enriched His-Pex3 as control. MBP-Inp1 C prevents binding of His-Pex3 to GST-Pex19. Protein was eluted from the amylose beads in 10 mM maltose buffer, and bound fractions were analyzed by SDS-PAGE and Coomassie staining of the gel (gel B). Because the bound His-Pex3 is not clearly distinguishable by Coomassie stain on gel B as a consequence of MBP-Inp1 breakdown bands, a Western blot with anti-His was done to show the presence of His-Pex3 bound to MBP-Inp1 C. M, molecular weight marker. Red arrow indicates His-Pex3. Protein visible on Gel B is 1/4 of total protein incubated with GST-Pex19. Protein visible on anti-His blot is 1/10. (B) atg36Δ cells were transformed with GFP-PTS1 and Inp1 under the control of the TPI1 promoter or an empty plasmid. The cells were then examined by epifluorescence microscopy. Scale bar, 5 µm.