Abstract
Atherosclerosis within two or more arterial beds has been termed “polyvascular disease”. Although polyvascular disease has long been associated with heightened cardiovascular risk, much is still unknown regarding its pathophysiology and management. In this past decade the field of cardiovascular disease has experienced exponential growth in terms of antithrombotic and lipid-lowering therapies aimed at mitigating ischemic events. This review describes the inherent risk associated with polyvascular disease in contemporary observational and clinical trial populations, and summarizes novel therapies in this high-risk population.
Introduction
The presence of atherosclerosis in ≥2 arterial beds is termed “polyvascular disease.” Polyvascular disease is not a new phenomenon, but a ubiquitous condition brought to the forefront in recent years through the expansion of clinical and research endeavors to include non-coronary atherosclerosis – specifically peripheral artery disease (PAD) and cerebrovascular disease (CVD). The relevance of polyvascular disease is centered on its associated heightened risk for cardiovascular (CV) death, myocardial infarction (MI), and ischemic stroke, a composite endpoint known as major adverse cardiovascular events (MACE).1 The present article highlights the inherent risks associated with polyvascular disease as described by contemporary data and summarizes pharmacologic therapies aimed at mitigating this risk.
Cardiovascular and Limb Risk in Polyvascular Disease
Observational Studies
CRUSADE
The advent of clinical registries capable of methodically collecting data have allowed investigators to evaluate polyvascular disease across large populations. The Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) was a dynamic registry of 111,972 patients presenting with a non-STE-segment elevation MI (NSTEMI). Overall, 13% of the study population had atherosclerosis in 2–3 vascular territories. In CRUSADE, an increased number of diseased arterial beds was associated with an increased rate in the composite endpoint of in-hospital mortality, MI, stroke, and congestive heart failure: 0 beds (9.9%), 1 bed (13.4%), 2 beds (18.9%), and 3 beds (21.9%) (P<0.0001). Furthermore, compared to patients without a history of atherosclerotic disease, investigators found a stepwise increased odds ratio of the composite endpoint of 1.07 (95% confidence interval [CI], 1.02–1.12), 1.25 (95% CI, 1.18–1.33), and 1.31 (95% CI, 1.15–1.46) in patients with disease in 1, 2, or 3 beds, respectively (Table 1).2
Table 1:
Studies of Polyvascular Disease
| Observational Studies | |||||
|---|---|---|---|---|---|
| Definition of Vascular Disease | Number of Affected Individuals | Risk of Polyvascular Disease | Benefits of Therapy in Individuals with Polyvascular Disease* | ||
| CRUSADE2 | CAD | A/S | 41,404 | ↑ In-hospital mortality, MI, stroke, or CHF | N/A |
| PAD | S | 11,345 | |||
| CVD | S | 9,973 | |||
| REACH1 | CAD | S | 26,397 | ↑ CV death, MI, or stroke | N/A |
| PAD | S | 5,872 | |||
| CVD | S | 12,810 | |||
| Randomized Clinical Trials | |||||
| CAPRIE3 | CAD | S | 6,302 | ↑ CV death, MI, or stroke | Clopidogrel vs. aspirin: ↓ CV death, MI, or stroke overall; no separate polyvascular disease analysis |
| PAD | S | 6,452 | |||
| CVD | S | 6,431 | |||
| TRA2°P-TIMI 504 | CAD | S | 17,779 | ↑ CV death, MI, or stroke | Vorapaxar vs. placebo: ↓ CV death, MI, or stroke; ↓ peripheral revascularization |
| PAD | S | 3,787 | |||
| CVD† | S | 4,883 | |||
| PEGASUS-TIMI 545 | CAD | S | 21,162 | ↑ CV death, MI, or stroke; ↑ CV death; ↑ MI; ↑ stroke | Ticagrelor + aspirin vs. aspirin alone: ↓ CV death, MI, or stroke; ↓ MALE |
| PAD | A/S | 1,143 | |||
| EUCLID6, 7 | CAD | S | 4,032 | ↑ CV death, MI, or stroke; ↑ lower extremity revascularization | Ticagrelor vs. clopidogrel: ↔ CV death, MI, or stroke |
| PAD | A/S | 13,885 | |||
| CVD | S | 1,650 | |||
| SAVOR-TIMI 538 | CAD | S | 10,278 | ↑ CV death, MI, or stroke | Saxagliptin vs. placebo: ↓ CV death, MI, or stroke overall; ↔ additional benefit in polyvascular subgroups |
| PAD | S | 1,959 | |||
| CVD | S | 2,094 | |||
| LEADER9 | CAD | A/S | 5,364 | ↑ CV death, MI, or stroke | Liraglutide vs. placebo: ↔ CV death, MI, or stroke |
| PAD | A | 1,066 | |||
| CVD | A/S | 1,968 | |||
| IMPROVE-IT10 | CAD | S | 18,144 | ↑ CV death, MI, or stroke | Ezetimibe vs. placebo: ↓ CV death, MI, or stroke overall; ↔ additional benefit in polyvascular subgroups |
| PAD | A/S | 1,005 | |||
| CVD | S | 1,071 | |||
| FOURIER11, 12 | CAD | S | 22,351 | ↑ CV death, MI, or stroke | Evolocumab vs. placebo: ↓ CV death, MI, or stroke; ↓ MALE |
| PAD | S | 3,642 | |||
| CVD | S | 5,337 | |||
| COMPASS13, 14 | CAD | S | 24,824 | ↑ CV death, MI, or stroke | Low-dose rivaroxaban+aspirin vs. aspirin+placebo: ↓ CV death, MI, or stroke; ↓ MALE |
| PAD | S | 4,129 | |||
| CVD | A/S | 1,919 | |||
Bold represents study therapy
Study discontinued early for individuals with ischemic stroke.
A=asymptomatic; CAD=coronary artery disease; CAPRIE=Clopidogrel vs. Aspirin in Patients at Risk of Ischemic Events; CHF = congestive heart failure; COMPASS=Cardiovascular Outcomes for People Using Anticoagulation Strategies; CRUSADE=Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines; CV=cardiovascular; CVD=cerebrovascular disease; EUCLID = Effects of Ticagrelor and Clopidogrel in Patients with Peripheral Artery Disease; FOURIER=Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk; IMPROVE-IT= mproved Reduction of Outcomes: Vytorin Efficacy International Trial; LEADER=Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Results; MALE=major adverse limb events; MI=myocardial infarction; PAD=peripheral artery disease; PEGASUS-TIMI 54=Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin; REACH=Reduction of Atherothrombosis for Continued Health; S=symptomatic; SAVOR-TIMI 53=Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-TIMI 53; TRA2°P-TIMI 50=Trial to Assess the Effects of Vorapaxar in Preventing Heart Attack and Stroke in Patients With Atherosclerosis-TIMI 50
REACH
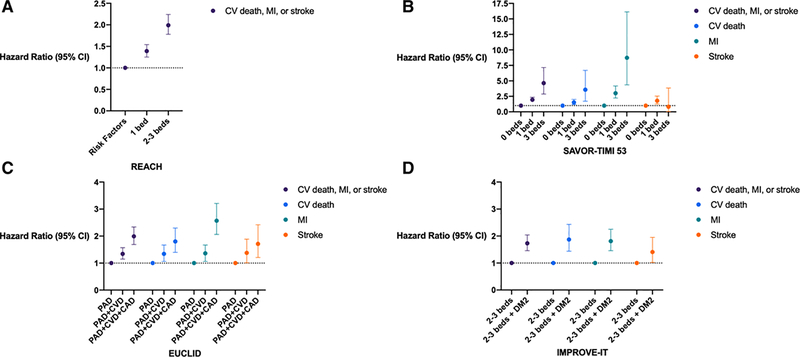
Our understanding of polyvascular disease was further advanced through the Reduction of Atherothrombosis for Continued Health (REACH) Registry, an international registry including patients ≥45 years of age with established CAD, CVD, PAD, or ≥3 risk factors for atherosclerotic disease (Table S1). Among 68,236 patients, the strongest prognosticator for future ischemic events in REACH was polyvascular disease, which was associated with a 99% increased risk of MACE at 4-year follow-up (P<0.001). This risk was greater than the increased risk of MACE associated with diabetes (44%, P<0.001) and prior cardiac ischemic events (71%, P<0.001) (Table 1).1 Based on estimates regarding the untoward CV risk associated with polyvascular disease in both CRUSADE and REACH, clinicians should enhance preventive therapies in this patient population.
Clinical Trials
CAPRIE
As novel antithrombotic therapies emerged over the past decades, emphasis was placed on identifying high-risk populations in efforts to maximize ischemic risk reduction and offset increased risk of bleeding. Patients with polyvascular disease represented an ideal cohort to enroll in such clinical trials not only to help “risk enrich” the study population, but also provide a platform to study this high-risk patient population. An early example of such a study is the Clopidogrel vs. Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial, which randomized 19,185 patients with recent MI, recent ischemic stroke, or symptomatic PAD to either clopidogrel (75 mg once daily) or aspirin (325 mg once daily) (Table S1). The rate of MACE among polyvascular disease patients randomized to aspirin was 20% – a number significantly greater than the entire aspirin cohort at 14% (Table 1). Furthermore, the risk of MACE was found to increase in parallel with the number of diseased vascular beds.3
TRA2°P-TIMI 50
The Trial to Assess the Effects of Vorapaxar in Preventing Heart Attack and Stroke in Patients With Atherosclerosis – Thrombolysis in Myocardial Infarction 50 (TRA2°P-TIMI 50) randomized 26,449 patients with stable prior MI, ischemic stroke, or PAD to either the protease-activated receptor 1 (PAR-1) antagonist vorapaxar or placebo (Table S1). By design, TRA2°P-TIMI 50 enrolled approximately 15% of its study population with a qualifying diagnosis of PAD or prior stroke. The rate of MACE at 3 years follow-up increased in stepwise fashion with each additional diseased arterial bed: 1 bed (7.8%), 2 beds (14.7%), and 3 beds (21.7%) (Table 1).4
PEGASUS-TIMI 54
The Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin (PEGASUS)-TIMI 54 trial randomized 21,162 patients with prior MI to ticagrelor or placebo on a background of low dose aspirin. At 3 years of follow-up, patients with CAD+PAD randomized to placebo experienced significantly higher rates of MACE compared to those without PAD (19.3% vs. 8.4%, respectively; HR 1.60; 95% CI, 1.20–2.13) (Table 1). The rates of the individual components of MACE were all higher in CAD+PAD patients.5
EUCLID
The majority of clinical trials have evaluated antithrombotic agents for secondary prevention in CAD populations. However, the Effects of Ticagrelor and Clopidogrel in Patients with Peripheral Artery Disease (EUCLID) trial exclusively enrolled patients with PAD. 13,885 patients with lower extremity PAD were randomized to either ticagrelor or clopidogrel and followed for a median of 30 months. Patients with PAD+CAD experienced higher rates of MACE compared to PAD alone (15.3% vs 8.9%, respectively; HR 1.28; 95% CI, 1.13–1.99).6 A subsequent analysis found nearly half of randomized patients had polyvascular disease: 2,639 (19%) had PAD+CAD; 2,049 (15%) had PAD+CVD; and 1,393 (10%) had PAD+CAD+CVD. Compared to patients with PAD alone, polyvascular disease was associated with a significant increase in MACE: PAD+CVD (HR 1.34; 95% CI, 1.15–1.57); PAD+CAD (HR 1.65; 95% CI, 1.43–1.91); and PAD+CAD+CVD (HR 1.99; 95% CI, 1.69–2.34). A finding particular to EUCLID was that polyvascular disease was independently associated with an increased risk of lower extremity revascularization (Table 1).7
In EUCLID, there was no significant difference in the rates of MACE or acute limb ischemia (ALI) with ticagrelor vs. clopidogrel. Analyses of primary outcomes according to polyvascular disease phenotype (PAD alone, PAD+CAD, PAD+CVD, or PAD+CAD+CVD) found no significant difference in rates of MACE or major bleeding.7 Overall, given the bleeding risk of DAPT with ticagrelor and the drug’s relative lack of additional benefit compared to clopidogrel in EUCLID, routine administration of ticagrelor in stable patients with polyvascular disease is likely not warranted. However, there may be benefit among certain high risk populations such as those with prior MI.5
Polyvascular Disease and Diabetes
SAVOR-TIMI 53
Because polyvascular disease and type 2 diabetes mellitus (T2DM) often coexist, these two prevalent conditions have also been closely studied together. The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR) – TIMI 53 trial randomized 16,492 T2DM patients with or at risk for CV disease to saxagliptin, a dipeptidyl peptidase-4 inhibitor, or placebo. At a median follow-up of 2.1 years, MACE occurred most often among patients with polyvascular disease: single bed disease (7.5%), 2-bed disease (15.6%), and 3-bed disease (23.9%, P<0.0001) (Table S1). After adjustment for differences in key baseline variables and compared to patients without any established atherosclerotic disease, a graded step-wise increase in MACE was observed with each additional diseased bed: 1 bed (HR 1.95; 95% CI, 1.63–2.35); 2 beds (HR 3.54; 95% CI, 2.84–4.43); and 3 beds (HR 4.64; 95% CI, 2.87–7.15) (Table 1). The rates of MACE were similar among diabetic patients randomized to saxagliptin vs. placebo across all three polyvascular disease cohorts.8
LEADER
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Results (LEADER) trial randomized 9,320 patients with T2DM and high CV risk to the glucagon-like peptide 1 analogue liraglutide or placebo. In LEADER, 1,536 (23%) had polyvascular disease, and 5,239 (77%) had single bed disease (Table S1). At a median follow-up of 3.8 years, the rate of MACE occurred more frequently in patients with polyvascular disease (22.2%) vs. single bed disease (15.3%) (HR 1.52; 95% CI, 1.33–1.72). The risk of MACE was non-significantly reduced among diabetic patients randomized to liraglutide vs. placebo (HR 0.82; 95% CI, 0.66–1.02) (Table 1).9
IMPROVE-IT
The Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) evaluated the addition of ezetimibe, a non-statin therapy limiting cholesterol absorption in the intestine, to statin therapy to mitigate ischemic events in patients with a recent ACS. IMPROVE-IT randomized 18,885 patients to simvastatin (40 mg daily) plus ezetimibe (10 mg day) or placebo. 1,930 (11%) had polyvascular disease, of which 737 (41%) had concomitant T2DM (Table S1). The rate of MACE at 7 years was 37.8% in patients with polyvascular disease vs. 19.5% without (HR 1.55; 95% CI, 1.41–1.70). In patients with polyvascular disease with and without diabetes the rate of MACE was 50.5% vs. 30.3%, respectively (HR 1.73; 95% CI, 1.46–2.04) (Table 1).10
As the aforementioned trials demonstrate, it is no longer sufficient to simply consider diabetes mellitus a precursor for atherosclerotic disease. Rather, diabetes and symptomatic atherosclerotic disease confer additive cardiovascular risk, and individuals with both diabetes and polyvascular disease represent one of the highest risk CV populations. This framework is not only important for clinical research but also patient care, and the presence of both comorbidities should be considered when clinicians weigh the risk, benefits, and cost of new therapies for these patients.
Pathophysiology of Polyvascular Disease
Basic science has long established atherosclerosis as a systemic process mediated through inflammation, cholesterol deposition, and thrombosis. This notion was further supported through post-mortem analyses which have shown that plaque deposition in coronary arteries is significantly associated with deposition in both carotid and femoral arteries. In addition, the severity of atherosclerotic disease in one major arterial bed is positively associated with disease in additional beds.15 Traditional cardiovascular risk factors, including tobacco use, hypertension, dyslipidemia, and diabetes, are strongly associated with polyvascular disease, suggesting a shared pathophysiology.16
Several lines of evidence, however, advocate that certain risk factors predispose individuals to specific polyvascular disease phenotypes. This is particularly true for PAD, where active tobacco use is considered a stronger risk factor for PAD than CAD.17, 18 Analyses have also shown no association between elevations in total cholesterol and low-density lipoprotein cholesterol (LDL-C) and incident PAD in women, which is in sharp contrast to the positive association with CAD and CVD.19, 20 Recent data instead suggest that components of atherogenic dyslipidemia, including elevated concentrations of triglyceride-rich lipoproteins, small, dense LDL particles, and low levels of high-density lipoprotein particles are associated with an increased incidence of PAD.21 Finally, patients with polyvascular disease have higher circulating concentrations of inflammatory markers, including interleukin-6 and high-sensitivity C-reactive protein.22
Screening for Polyvascular Disease
Although patients with polyvascular disease are at heightened risk for cardiovascular events, routine screening for polyvascular disease following detection of atherosclerosis in one arterial bed remains controversial. One such reason for this contention is that some atherosclerotic diseases, in particular PAD and CVD, are not consistently viewed by clinicians as conferring the same risk as CAD.23, 24 Another cause for clinical equipoise is the fact that guideline-recommended interventions for atherosclerotic disease do not change with the detection of an additional diseased bed. Furthermore, the cost-effectiveness of such screening remains unclear. As a result, current professional guidelines do not recommend routine screening to detect atherosclerosis in additional vascular beds, although screening may be considered for the purposes of risk stratification (Class IIb, level of evidence [LOE] B).25, 26 Screening may be of greater utility in patients undergoing arterial revascularization, such as in individuals undergoing elective coronary artery bypass grafting or carotid endarterectomy.26
Nonetheless, all patients with atherosclerotic disease should undergo a full history and physical exam with careful attention to signs and symptoms of disease in all major arterial beds. Many non-cardiac symptoms, such as claudication and amaurosis fugax, are often missed, and a detailed clinical assessment may help identify individuals in need of aggressive antithrombotic therapy or even revascularization. In addition, increased patient education regarding polyvascular disease and its associated heightened ischemic risk, may help serve as additional motivation for patients in terms of promoting medication adherence and lifestyle modification.
Medical Management of Polyvascular Disease
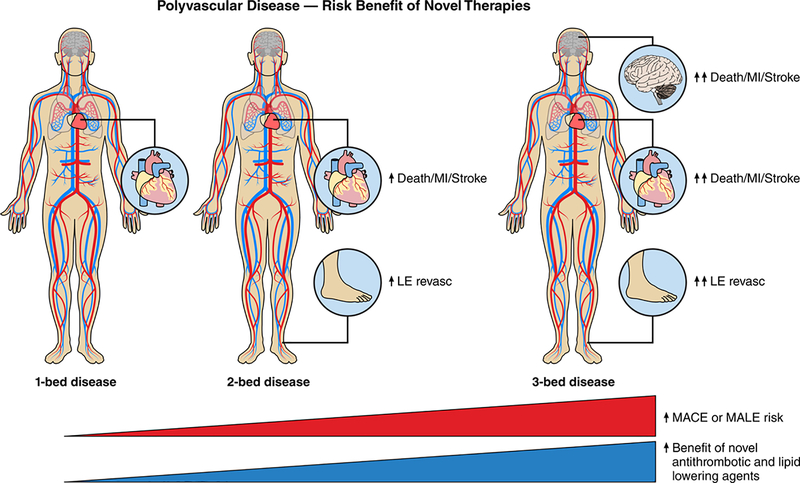
As presented above, patients with polyvascular disease represent a malignant atherothrombotic phenotype at increased risk of ischemic events (Figure 1). The majority of clinical trials studying atherosclerotic disease, however, do not typically report the efficacy of therapies according to polyvascular disease phenotype. Additionally, it is only within recent years that investigators have begun including limb-related outcomes to examine the full spectrum of cardiovascular outcomes. Furthermore, given that many novel antiplatelet and antithrombotic therapies are associated with an increased risk of bleeding, incorporating polyvascular disease into these trials is necessary to help clinicians balance the risks and benefits of emerging therapies. The following discussion highlights recently completed trials involving medical therapies aimed at reducing major adverse events (cardiac and limb-related) in the setting of polyvascular disease.
Figure 1. Risk of Major Adverse Cardiac Events in Polyvascular Disease.
Figures display hazard ratios and 95% confidence intervals of polyvascular disease in clinical registries and randomized controlled trials. A. REACH Registry data comparing cardiovascular risk factors alone to symptomatic atherosclerotic disease in a single versus 2–3 arterial beds B. SAVOR TIMI 53 Trial C. EUCLID Trial D. IMPROVE-IT Trial
CI, confidence interval; CV, cardiovascular; MI, myocardial infarction; PAD, peripheral artery disease; CVD; cerebrovascular disease; CAD, coronary artery disease; DM2, type 2 diabetes mellitus
FOURIER
The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial recruited 27,564 high-risk patients with CV disease including prior MI, ischemic stroke, or symptomatic PAD already receiving appropriate lipid lowering therapy (Table S1). Patients were randomized to evolocumab, a fully human monoclonal antibody against PCSK9, or placebo on a background of statin therapy. After a median follow-up of 2.2 years and in the overall study population, evolocumab led to a significant reduction in MACE (5.9% vs. 7.4%; HR 0.80; 95% CI, 0.73–0.88).11 Among patients with polyvascular disease, treatment with evolocumab was associated with a nonsignificant reduction in MACE (11.1% vs. 12.9%; HR 0.86; 95% CI, 0.71–1.04). It is important to note however that among those with PAD, of which 69% had a prior history of MI or stroke, evolocumab was associated with a significant decreased risk of MACE (HR 0.73; 95% CI 0.59–0.91) and a trend towards lower rates of major adverse limb events (HR 0.63; 95% CI, 0.39–1.03) (Table 1).12
COMPASS
Recently, investigators have begun testing direct oral anticoagulants for the secondary prevention of atherosclerotic disease. The Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial randomized 27,395 patients with stable atherosclerotic disease in a 1:1:1 double-blind fashion to rivaroxaban (2.5 mg twice daily) plus aspirin (100 mg daily), rivaroxaban (5 mg daily) alone, or aspirin (100 mg daily) alone. The trial included high risk individuals with CAD and/or PAD (Table S1). In terms of polyvascular disease, 17.9% of trial participants had a history of CAD+PAD and 7.0% had a history of PAD+carotid artery stenosis (Table 1).13
In the overall study population, rivaroxaban plus aspirin, when compared with aspirin alone, led to a 24% reduction in MACE (HR 0.76; 95% CI, 0.66–0.86). Combination therapy did lead to more major bleeding (3.1% vs. 1.9%; P<0.001). However, there were no statistically significant differences in fatal bleeding or intracranial hemorrhage. A net clinical benefit analysis, which encompassed CV death, stroke, MI, fatal bleeding, or symptomatic bleeding into a critical organ, found a 20% lower risk with rivaroxaban plus aspirin compared to aspirin alone (HR 0.80; 95% CI, 0.70–0.91).14
The results of COMPASS represent a significant breakthrough in pharmacologic therapy among high ischemic risk populations. Accordingly, both U.S. and Canadian federal drug agencies have since approved rivaroxaban 2.5 mg twice daily in combination with low-dose aspirin to reduce cardiovascular events among stable patients with CAD or PAD. Data for polyvascular disease subgroups in COMPASS are not yet available. However, as with other therapies, one would expect such patients to have a greater benefit of low-dose rivaroxaban than individuals with disease in a single vascular bed.
Intensification of Therapy in Polyvascular Disease
Recent advancements in pharmacologic therapies, primarily in the antithrombotic and antilipidemic arenas, have provided clinicians with the opportunity to intensify medical therapy in patients with polyvascular disease. At present, a major question for clinicians is when to utilize more potent antithrombotic therapies in patients with polyvascular disease. This decision centers on the balance between adverse ischemic event reduction and risks, in particular severe bleeding, provided by these therapies. It is the opinion of these authors that the bleeding risk associated with routine use of dual antiplatelet therapy outweighs the benefits in stable, asymptomatic polyvascular disease (Figure 2 Central Figure). In light of the recent COMPASS findings, however, anticoagulation with low-dose rivaroxaban (2.5 mg twice daily) in addition to aspirin should be considered in patients with polyvascular disease – in particular, in the setting of symptomatic PAD, prior lower extremity revascularization or diabetes.13, 14 In patients with a prior MI as well as symptomatic PAD, an alternative regimen of low-dose aspirin and ticagrelor is also a reasonable.5
Figure 2. Polyvascular Disease and use of Novel Therapies (Central Figure).
MACE = major adverse cardiac events
Another clinical conundrum for patients with polyvascular disease is when to consider intensification of lipid therapy. Although both evolocumab and alirocumab have been approved by the FDA for individuals with atherosclerotic cardiovascular disease, the barriers from insurers to cover such therapies remain significant.27, 28 Therefore, the dilemma for clinicians is often whether the therapy is economically feasible for patients. Our practice is to prescribe high-intensity statin therapy for all individuals with polyvascular disease, targeting an LDL-C <70 mg/dL. If unable to achieve this reduction in LDL-C with statins alone, the edition of ezetimibe should be considered. Lastly, if still unable to achieve this threshold, we recommend PCSK9 inhibition.29
Conclusions
Reappraisal of the current clinical landscape regarding polyvascular disease yields the following conclusions. First, focused recruitment of patients with polyvascular disease in clinical trials is essential to further our understanding of the pathophysiology and inherent risk of specific polyvascular disease phenotypes, in particular those with CVD as this is the least studied cohort. Second, patients with polyvascular disease, in particular those with CAD and/or PAD, are among the highest risk for atherothrombosis, and this untoward risk is further augmented in the setting of diabetes. Clinicians should endeavor to maximize the use of preventive therapies endorsed by societal guidelines in this population. Third, current professional guidelines primarily recommend therapies based on individual vascular beds. Highlighting the polyvascular phenotype within the framework of future guideline iterations may improve both provider and patient awareness and help guide clinical practice. Lastly, the heightened ischemic risk associated with polyvascular disease warrants consideration of treatment with novel more potent antithrombotic or lipid-lowering therapies to mitigate ischemic events.
Supplementary Material
Acknowledgments
Disclosures
Aday: Reports no conflicts.
Gutierrez: Consulting fees from Janssen Pharmaceuticals, Inc.
Patel: Consulting fees from Bayer, Ortho-McNeil-Janssen, theheart.og, Ikaria, and Duke CME; research grants from AstraZeneca, CSI, HeartFlow, Janssen Research & Development, Maquet, Medtronic, and the NHLBI.
Jones: Research grants to the DCRI from the American Heart Association, AstraZeneca, Boston Scientific, and Bristol‐Myers Squibb.
References
- 1.Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D’Agostino R, Liau CS, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010; 304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt DL, Peterson ED, Harrington RA, Ou FS, Cannon CP, Gibson CM, Kleiman NS, Brindis RG, Peacock WF, Brener SJ, et al. Prior polyvascular disease: risk factor for adverse ischaemic outcomes in acute coronary syndromes. Eur Heart J. 2009; 30:1195–1202. [DOI] [PubMed] [Google Scholar]
- 3.Hirsh J, Bhatt DL. Comparative benefits of clopidogrel and aspirin in high-risk patient populations: lessons from the CAPRIE and CURE studies. Arch Intern Med. 2004; 164:2106–2110 [DOI] [PubMed] [Google Scholar]
- 4.Bonaca MP, Creager MA, Olin J, Scirica BM, Bohula EA, Murphy SA, Braunwald E, Morrow DA. Vorapaxar reduces peripheral revascularization regardless of number of diseased territories: insights from the TRA2°P-TIMI 50 trial. American College of Cardiology Scientific Sessions. 2013. [Google Scholar]
- 5.Bonaca MP, Bhatt DL, Storey RF, Steg PG, Cohen M, Kuder J, Goodrich E, Nicolau JC, Parkhomenko A, Lopez-Sendon J, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. 2016; 67:2719–2728. [DOI] [PubMed] [Google Scholar]
- 6.Berger JS, Abramson BL, Lopes RD, Heizer G, Rockhold FW, Baumgartner I, Fowkes FGR, Held P, Katona BG, Norgren L, et al. Ticagrelor versus clopidogrel in patients with symptomatic peripheral artery disease and prior coronary artery disease: Insights from the EUCLID trial. Vasc Med. 2018; 6:523–530. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez JA, Mulder H, Jones WS, Rockhold FW, Baumgartner I, Berger JS, Blomster JI, Fowkes GR, Held P, Katona BG, et al. Polyvascular disease and risk of major adverse cardiovascular events in peripheral artery disease: A secondary analysis of the EUCLID trial. JAMA Netw Open. 2018;1:e185239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez JA, Scirica BM, Bonaca MP, Steg PG, Mosenzon O, Hirshberg B, Im K, Raz I, Braunwald E, Bhatt DL. Prevalence and outcomes of polyvascular (coronary, peripheral, or cerebrovascular) disease in patients with diabetes mellitus (From the SAVOR-TIMI 53 Trial). Am J Cardiol. 2019; 123:145–152. [DOI] [PubMed] [Google Scholar]
- 9.Verma S, Bhatt DL, Bain SC, Buse JB, Mann JFE, Marso SP, Nauck MA, Poulter NR, Pratley RE, Zinman B, et al. Effect of liraglutide on cardiovascular events in patients with type 2 diabetes mellitus and polyvascular disease: results of the LEADER trial. Circulation. 2018; 137:2179–2183. [DOI] [PubMed] [Google Scholar]
- 10.Bonaca MP, Gutierrez JA, Cannon C, Giugliano R, Blazing M, Park JG, White J, Tershakovec A, Braunwald E. Polyvascular disease, type 2 diabetes, and long-term vascular risk: a secondary analysis of the IMPROVE-IT trial. Lancet Diabetes Endocrinol. 2018; 6:934–943. [DOI] [PubMed] [Google Scholar]
- 11.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and clinical outcomes in patients with cardiovascular Ddsease. N Engl J Med. 2017; 376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 12.Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, Kuder J, Murphy SA, Jukema JW, Lewis BS, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial. Circulation. 2018; 137:338–350. [DOI] [PubMed] [Google Scholar]
- 13.Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018; 391:219–229. [DOI] [PubMed] [Google Scholar]
- 14.Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017; 377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 15.Vidakovic R, Schouten O, Kuiper R, Hoeks SE, Flu WJ, van Kuijk JP, Goei D, Verhagen HJ, Neskovic AN, Poldermans D. The prevalence of polyvascular disease in patients referred for peripheral arterial disease. Eur J Vasc Endovasc Surg. 2009; 38:435–440. [DOI] [PubMed] [Google Scholar]
- 16.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015; 116:1509–1526. [DOI] [PubMed] [Google Scholar]
- 17.Conen D, Everett BM, Kurth T, Creager MA, Buring JE, Ridker PM, Pradhan AD. Smoking, smoking cessation, [corrected] and risk for symptomatic peripheral artery disease in women: a cohort study. Ann Intern Med. 2011; 154:719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu JT, Creager MA. The relationship of cigarette smoking to peripheral arterial disease. Rev Cardiovasc Med. 2004; 5:189–193. [PubMed] [Google Scholar]
- 19.Pradhan AD, Shrivastava S, Cook NR, Rifai N, Creager MA, Ridker PM. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation. 2008; 117:823–831. [DOI] [PubMed] [Google Scholar]
- 20.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009; 119:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aday AW, Lawler PR, Cook NR, Ridker PM, Mora S, Pradhan AD. Lipoprotein particle profiles, standard lipids, and peripheral artery disease incidence. Circulation. 2018; 138:2330–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, Koenig W, Siegbahn A, Steg PG, Soffer J, et al. Inflammatory Biomarkers Interleukin-6 and C-Reactive Protein and Outcomes in Stable Coronary Heart Disease: Experiences From the STABILITY trial. J Am Heart Assoc. 2017;6:e005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001; 286:1317–1324. [DOI] [PubMed] [Google Scholar]
- 24.McDermott MM, Hahn EA, Greenland P, Cella D, Ockene JK, Brogan D, Pearce WH, Hirsch AT, Hanley K, Odom L, et al. Atherosclerotic risk factor reduction in peripheral arterial disease: results of a national physician survey. J Gen Intern Med. 2002; 17:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017; 135:e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018; 39:763–816. [DOI] [PubMed] [Google Scholar]
- 27.Baum SJ, Toth PP, Underberg JA, Jellinger P, Ross J, Wilemon K. PCSK9 inhibitor access barriers-issues and recommendations: Improving the access process for patients, clinicians and payers. Clin Cardiol. 2017; 40:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doshi JA, Puckett JT, Parmacek MS, Rader DJ. Prior authorization requirements for proprotein convertase subtilisin/kexin type 9 inhibitors across US private and public payers. Circ Cardiovasc Qual Outcomes. 2018; 11:e003939. [DOI] [PubMed] [Google Scholar]
- 29.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, Ferranti Sd, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/ APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019. 18; 139: e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.