Abstract
Alcoholic beverages with low ethanol contents were produced by fermenting black currant juice with Saccharomyces and non-Saccharomyces yeasts without added sugar. The effects of different yeasts on the phenolic compounds (anthocyanins, hydroxycinnamic acids, flavonols, and flavan-3-ols) and other selected constituents (the ethanol content, residual sugars, organic acids, and color) of the black currants were assessed. Single yeast-fermented beverages had higher ethanol contents (3.84–4.47%, v/v) than those produced by sequential fermentation. In general, the fermentation of black currant juice increased the contents of organic acids and flavonols, whereas anthocyanin contents decreased. All of the fermentations decreased the contents of glycosylated nitrile-containing hydroxycinnamic acids, resulting in higher contents of the corresponding aglycons. Fermentation with Saccharomyces bayanus resulted in lower anthocyanin and organic acid contents compared to the other yeasts. Sequential fermentations with Saccharomyces cerevisiae and Metschnikowia pulcherrima led to the highest total hydroxycinnamic acids and anthocyanins among all of the fermentations.
Keywords: black currant, fermentation, Saccharomyces yeast, non-Saccharomyces yeast, phenolic compounds
1. Introduction
Black currant (Ribes nigrum) is the third most cultivated berry crop in Finland and the second largest berry crop in Europe. The phenolic composition of black currants has been widely studied and varies according to the cultivar, growth location, and weather conditions.1,2 The rich phenolic contents of the berries affect their sensory properties, resulting in a bitter taste and more astringency.3 The phenolic compounds in black currants also have potential beneficial effects on human health.4,5 Anthocyanins are the primary group of phenolic compounds in black currants, followed by flavonols, flavan-3-ols, and phenolic acids.6,7
Wine yeasts have a comprehensive effect on the sensory quality of final wine products, and therefore, selecting the yeast is an important task.8−10Saccharomyces cerevisiae and Saccharomyces bayanus are the most frequently used wine yeast species as a result of their high ethanol tolerance and good fermentation performance in matrices with high sugar contents and low pH values. Non-traditional wine yeasts, such as Torulaspora delbrueckii, Metschnikowia pulcherrima, and Metschnikowia fructicola, have become increasingly popular in research on fruit wine fermentation in recent years, and these yeasts are commercially available for fine production at an industrial scale as well. Non-Saccharomyces wine yeasts are often used to improve the comprehensive quality parameters, such as increasing the formation and/or release of aromatic volatiles,11 and, thus, possibly improve the sensory properties,8 reduce the alcohol content,9 and improve the stability of the anthocyanin pigments.10 As a result of their relatively low fermentation performance and ethanol tolerance, non-Saccharomyces yeasts are primarily used in mixed or sequential fermentations with S. cerevisiae.
Yeast fermentation is known to affect the composition of anthocyanins and pyranoanthocyanins, a group of anthocyanins primarily present in alcoholic beverages. They are anthocyanin derivatives formed by condensation between anthocyanins and yeast metabolites, such as pyruvic acid, acetaldehyde, and vinylphenols. The color of pyranoanthocyanins is usually reddish brown or reddish orange, and their typical maximal absorption wavelength is typically between 495 and 515 nm,12 lower than those of the corresponding monomeric anthocyanins. Pyranoanthocyanins are formed during the wine fermentation mostly when 20–85% of the glucose is consumed.13 However, their formation is a slow process that takes more time than sugar fermentation.11 The formation of some pyranoanthocyanins requires the presence of oxidants to be completed.13
During fermentation, yeasts produce intra- and extracellular enzymes, such as pectinases, esterases, proteases, and glycosidases, which have effects on the technological and sensory properties of the final wine products.14,15 The production rate of the enzymes depends upon the yeast species and strain.14−16 Pectinases degrade pectin, which results in improved clarification and filtration as well as the release of phenolic compounds, such as anthocyanins and flavonols, in addition to flavor compounds.16 β-Glucosidase is a common glycosidase produced by the wine yeasts. It can increase or decrease the final quality of a wine by releasing glycoside moieties from terpenoids (increasing the aroma complexity17) and anthocyanins (decreasing the color intensity15).
The effects of processing, such as heat, enzymatic treatment,3,18,19 and storage,20 on phenolic compounds and, thus, the sensory properties of black currants have been widely studied. However, there are only a few studies focused on the effects of yeast fermentation on the chemical composition of black currant beverages.21−27 Moreover, no study reporting on comparisons of the impacts of different yeasts on the chemical composition of fermented black currant were found. In the present study, we aimed to assess comprehensive effects of pure fermentation with single yeast strains of S. cerevisiae, S. bayanus, or T. delbrueckii yeasts as well as sequential fermentations of M. pulcherrima and M. fructicola with S. cerevisiae on the chemical composition of black currant juice. Special focus was directed toward the effects on various non-volatile constituents, such as phenolic compounds, sugars, and organic acids, as well as ethanol, which are known to impact the sensory properties of beverages strongly. Black currant pulp containing a high amount of pectin was used in this study because its flavor properties resemble those of the original black currants more than those of the enzymatically treated black currants.28 Additionally, sugars were not added to the juice before or during fermentation because our goal was to produce beverages with a low ethanol concentration instead of fruit wines.
2. Materials and Methods
2.1. Yeasts and Standard Compounds
The two S. cerevisiae strains W15 and ICV-K1 (Sc1 and Sc2, Lalvin, Montreal, Quebec, Canada), T. delbrueckii Biodiva (Td, Level, Edwardstown, Australia), M. pulcherrima Flavia (M1, Level, Edwardstown, Australia), and M. fructicola IOC Gaïa (M2, Edwardstown, Australia) were kindly provided by Lallemand, Inc. (Montreal, Quebec, Canada), and S. bayanus (Sb, Condessa, Viinitialo Melkko, Ltd., Lahti, Finland) was purchased from a local wine equipment store in Turku, Finland.
Quercetin-3-O-glucoside, cyanidin-3-O-glucoside, and (+)-catechin were purchased from Extrasynthese (Genay, France). p-Coumaric acid, chlorogenic acid, xylitol, tartaric acid, quinic acid, fumaric acid, shikimic acid, and galacturonic acid were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Citric acid and sucrose were purchased from J.T. Baker Chemicals (Leuven, Belgium). n-Butanol was purchased from Riedel-de Haën (Morris Plains, NJ, U.S.A.), and ethanol was purchased from Altia (Helsinki, Finland).
2.2. Preparation of Black Currant Juice and Fermented Beverages
Frozen Finnish black currants (Pakkasmarja, Ltd., Suonenjoki, Finland) were purchased from a local supermarket. All of the black currants used here were from the same production batch. They were stored at −20 °C until further use. Before the juice pressing, the black currants were thawed in the microwave oven for 1.5 min at 350 W, mixed, and thawed again for 1.5 min at 350 W. Thawed berries were cold-pressed with a vertical tincture press (HP 2 H, Hafico, Fischer Maschinenfabrik, Neuss, Germany) at a pressure of 140 kg/cm3. The black currant juice was pasteurized in 50 mL glass bottles by immersing the bottles into boiling water. The temperature changes in the juice were followed with a thermometer (TM-947SD, Lutron Electronic Enterprice Co., Ltd., Taipei, Taiwan) until it reached 97 °C, kept at 97 °C for 30 s, and then immediately transferred to ice to cool to 25 °C. For each fermentation, 100 mL of pasteurized juice was measured and transferred to a glass bottle.
Before inoculation of the black currant juice, all of the yeasts were reactivated with water rehydrant solution (Go-Ferm, Lallemand, Inc., Montreal, Quebec, Canada) to shorten the lag phase of the fermentation. The optimal temperature and time were used to reactivate each yeast, which were as follows: S. cerevisiae 1, 35–40 °C; S. cerevisiae 2, 40 °C; S. bayanus, 30–35 °C; T. delbrueckii, 30 °C; M. pulcherrima, 30 °C; and M. fructicola, 20–30 °C. The minimum reactivation time was 20 min for every yeast but less than 45 min. The inoculation was performed so that the inoculation amount was 0.25 g/L of active dried yeast, which corresponds to 1.4 × 108 colony-forming units (CFU)/mL of S. cerevisiae 1, 3.6 × 109 CFU/mL of S. cerevisiae 2, 9.7 × 107 CFU/mL of S. bayanus, 4.7 × 108 CFU/mL of T. delbrueckii, 7.3 × 108 CFU/mL of M. pulcherrima, and 1.2 × 108 CFU/mL of M. fructicola. The cell populations of each yeast were determined by plate counting on YPD agar (1% yeast extract, 2%, peptone, 2% dextrose, and 2% agar). The plates were incubated at 35 °C for 48–72 h before counting. The fermentation was performed at 21 °C in the dark. For sequential fermentations, the juice was pre-fermented with Metschnikowia yeasts for 24 h, and after that, S. cerevisiae yeast was added to continue the fermentation, as described above. All of the fermentations were performed in triplicate.
The fermentations were monitored by measuring the Brix (Hanna Instruments, Woonsocket, RI, U.S.A.) regularly and were stopped by adding yeast killer (potassium sulfate/potassium sorbate, 1:1, Jässtopp D, Viinitialo Melkko, Lahti, Finland) when the Brix had remained constant for 24 h. The beverages were centrifuged at 1500g for 10 min, and the supernatant was collected and stored in the freezer at −80 °C.
2.3. Extraction of Phenolic Acids and Flavonol Glycosides
To extract the non-anthocyanin phenolic compounds, 2 g of each sample was weighed in an extraction tube and 5 mL of ethyl acetate was added. Thereafter, the extraction tube was shaken vigorously for 90 s, followed by centrifugation for 5 min at 1500g. The supernatant was collected in an evaporation flask. The extraction was repeated 4 times, and the extracts were combined. Ethyl acetate was evaporated to dryness at 35 °C under a vacuum with a rotary evaporator (Heidolph Instruments, Schwabach, Germany), and the extract was reconstituted with 1 mL of methanol and filtered through a 0.22 μm polytetrafluoroethylene (PTFE) filter. The extractions were performed in triplicate.
2.4. Determination of Phenolic Acids, Flavonols, and Anthocyanins
The phenolic acids, flavonol glycosides, flavan-3-ols, methyl citrates, and anthocyanins were determined using an ultra-high-performance liquid chromatography (UHPLC) instrument (Nexera 30 Series, Shimadzu Corp., Kyoto, Japan) coupled with a SPD-M20A diode array detector (DAD), as described previously by Mäkilä et al.,20 with slight modifications. The mobile phases were water (A) and acetonitrile (B), both containing formic acid (0.1%, v/v). The phenolic acids, flavonols, flavan-3-ols, and methyl citrates were separated with the following mobile phase B gradient: 0–15 min, 2–18%; 15–20 min, 18%; 20–30 min, 18–20%; 30–35, 20–60%; and 40–45 min, 60–2%. The flow rate of the mobile phase was 1 mL/min. The separation of compounds was performed with an AerisPeptide column (XB-C18, 150 mm × 4.6 mm × 3.6 μm, Phenomenex, Torrance, CA, U.S.A.). The oven temperature was set to 25 °C. Flavan-3-ols and methyl citrates were recorded at 280 nm; phenolic acids were recorded at 310 and 320 nm; and flavonol glycosides were recorded at 360 nm. (+)-Catechin, p-coumaric acid, chlorogenic acid, and quercetin-3-O-glucoside were used to quantify the compounds at 280, 310, 320, and 360 nm, respectively. Five-point calibration curves were constructed using reference compounds over a concentration range of 0.006–0.40 g/L.
Before the anthocyanin analysis, 500 μL of black currant juice or fermented beverage was diluted with 500 μL of acidified methanol (using concentrated HCl at 1%, v/v) and filtered through a 0.22 μm PTFE filter. The mobile phases were water (A) and acetonitrile (B), both containing formic acid (5%, v/v). The following gradient was used for the mobile phase B: 0–10 min, 5–10%; 10–15 min, 10—15%; 15–20 min, 15–40%; 20–30 min, 40–90%; and 30–35 min, 90–5%. The flow rate was 1 mL/min. The separation of the compounds was performed with the same column as mentioned previously. The oven temperature was 36 °C, and the peaks were recorded at 520 nm. Cyanidin-3-O-glucoside was used to construct a five-point calibration curve over a concentration range of 0.021–0.34 g/L to quantify the anthocyanins.
2.5. Qualitative Analysis of Phenolic Acids, Flavonols, and Anthocyanins
The phenolic acids, flavonol glycosides, and anthocyanins were identified with an Elute UHPLC–DAD instrument coupled with an Impact II ESI–QTOF system (Bruker Daltonik GmbH, Bremen, Germany). The system was controlled with Compass HyStar software (Bruker Daltonik GmbH, Bremen, Germany). The same mobile phases A and B were used as those described previously. In the analysis of flavan-3-ols, phenolic acids, and flavonols, the following mobile phase B gradient was used: 0–9.4 min, 2–18%; 9.4–11.80 min, 18%; 11.80–14.20 min, 18–20%; 14.20–16.50 min, 20–60%; 16.50–18.90 min, 60–2%; and 18.9–21.20 min, 2%. The flow rate was 0.5 mL/min. The compounds were separated with a bioZen column (XB-C18, 150 mm × 2.1 mm × 1.7 μm, Phenomenex, Torrance, CA, U.S.A.). The oven temperature was set to 35 °C, and the peaks were recorded at 310, 320, and 360 nm. The mass spectrometer was operated in positive- and negative-ion modes. The capillary voltage was set to 4.5 kV; the nebulizer pressure was set to 4.8 bar; the dry gas flow was set to 12 L/h; and the dry temperature was set to 350 °C. The ions were scanned over a range of m/z 20–1000.
The chromatographic conditions and column used for the qualitative anthocyanin analysis were the same as those used previously for quantitative analysis. A flow of 0.140 μL/min was directed to the mass spectrometer operated in positive- and negative-ion modes. The capillary voltage was 4.5 kV; the nebulizer pressure was 1.4 bar; the dry gas flow was 9.0 L/h; and the dry temperature was 230 °C. The ions were scanned over a range of m/z 20–1000.
2.6. Determination of Sugars and Organic Acids
The sugars and organic acids were analyzed with gas chromatography (GC, GC-2010Plus, Shimadzu Corp., Kyoto, Japan) equipped with a flame ionization detector (FID) as trimethylsilyl (TMS) derivatives as described before in our previous study.29 The compounds were quantified using the internal standards: xylitol for sugars and tartaric acid for acids at a concentration of 5 g/L. External standards (quinic, fumaric, shikimic, citric, and galacturonic acids, all at 5 g/L) were analyzed to identify and calculate the correction cofactors.
2.7. Ethanol Determination in Fermented Beverages
The ethanol was determined with GC (GC-2010Plus, Shimadzu Corp., Kyoto, Japan) equipped with a flame ionization detector (FID) as described before by Liu et al.30 Before the analysis, the samples were filtered with 0.45 μm RC filters. 2-Butanol was added as an internal standard for ethanol quantification.
2.8. Determination of Color Properties
The color properties were determined from undiluted samples by spectrophotometric measurements (Evolution UV–vis 300, Thermo Fisher Scientific, Wilmington, DE, U.S.A.) using a plastic cell with a 1 cm optical pathway. The absorbances were recorded at 420, 520, and 620 nm. According to Liu et al.,30 percentages of yellow (% yellow), red (% red), and blue (% blue) and the color intensity (CI) and color tonality (CT) were calculated as follow:
2.9. Statistical Analysis
SPSS 25.0.0.1. (IBM SPSS Statistics, Inc., Chicago, IL, U.S.A.) used a one-way analysis of variance (ANOVA) with Tukey’s test and an independent sample t test. The one-way ANOVA was used to determine the statistical difference between different yeast fermentations, and the independent sample t test was used to determine the differences between the black currant juice and the average fermented beverage. Unscrambler X (version 11, CAMO, Inc., Oslo, Norway) was used to performe principal component analysis (PCA1). PCA1 was constructed using one black currant juice sample, three biological replicates of seven fermented beverage samples, and 57 chemical variables. The samples were grouped by juice and the yeast species into seven groups. PCA2 was constructed using three biological replicates of seven fermented beverage samples, and there were 57 chemical variables. The samples were grouped by the yeast species.
3. Results and Discussion
3.1. Fermentation Kinetics
The fermentation times between the single yeast and sequential fermentations were notably different. Sequential fermentations with M. pulcherrima (M1) or M. fructicola (M2) and the S. cerevisiae strains (Sc1 and Sc2) lasted only 6 days, whereas the single yeast fermentations took 8–11 days to complete. The fermentation kinetics measured as changes in Brix values are presented in Figure 1. The Brix values of the M1Sc2 and M2Sc1 fermentations also decreased less than in other fermentations according to the initial Brix of the juice. The single yeast fermentation with S. bayanus (Sb) had the longest lag phase, and its fermentation lasted the longest. Similar low fermentation rates for S. bayanus have been reported before.31
Figure 1.
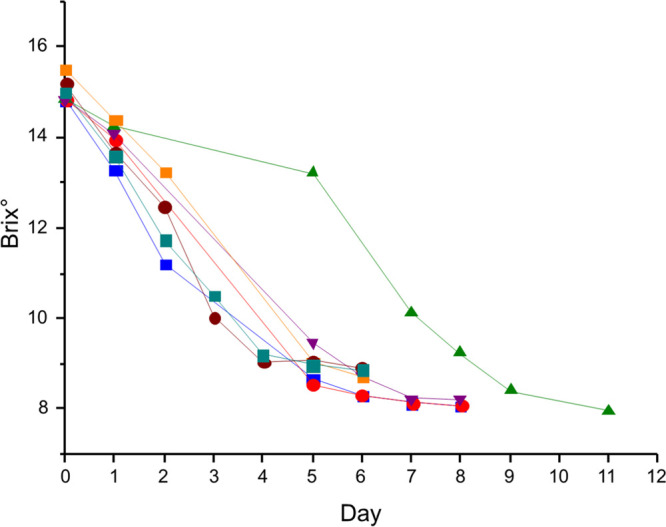
Decrease in Brix values during the fermentation: (blue ■) S. cerevisiae 1, (red ●) S. cerevisiae 2, (green ▲) S. bayanus, (purple ▼) T. delbrueckii, (orange ■) M. pulcherrima + S. cerevisiae 1, (cyan ■) M. fructicola + S. cerevisiae 1, and (maroon ●) M. pulcherrima + S. cerevisiae 2.
The pH was measured before and after each fermentation (Table 1). The initial pH of the black currant juice was 2.96, and after fermentation the values varied between 2.93 (M2Sc1) and 3.07 (Sb). The S. bayanus and T. delbrueckii (Td) fermentations increased the pH significantly compared to the black currant juice. The increase in the pH cannot be explained by the compounds determined in this study, but, for example, changes in the pectin concentration could have an effect on the pH.32
Table 1. Physicochemical Parameters of Black Currant Juice and Fermented Beveragesa.
| number | juice | Sc1 | Sc2 | Sb | Td | M1Sc1 | M1Sc2 | M2Sc1 | averaged beverageb | |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 2.96 ± 0.05 ab | 2.95 ± 0.00 a | 2.99 ± 0.01 abc | 3.07 ± 0.02 c | 3.06 ± 0.01 c | 3.00 ± 0.04 abc | 2.93 ± 0.01 ab | 3.03 ± 0.05 bc | 3.00 ± 0.05 | |
| ethanol (%, v/v) | nd | 3.84 ± 0.3 ab | 4.47 ± 0.4 c | 4.17 ± 0.4 bc | 4.07 ± 0.5 bc | 3.69 ± 0.4 ab | 3.46 ± 0.2 a | 3.79 ± 0.4 ab | 3.9 ± 0.5c | |
| Organic Acids (g/L) | ||||||||||
| 1A | succinic acid | nd | 0.7 ± 0.06 b | 0.7 ± 0.02 b | 0.9 ± 0.08 c | 0.8 ± 0.03 b | 0.5 ± 0.02 a | 0.5 ± 0.2 a | 0.5 ± 0.03 a | 0.6 ± 0.2 |
| 2A | malic acid | 3.1 ± 0.4 | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.6 ± 0.2 | 2.8 ± 0.06 | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.6 ± 0.2 | 2.7 ± 0.4 |
| 3A | shikimic acid | 0.4 ± 0.03 cd | 0.4 ± 0.01 ab | 0.4 ± 0.01 abc | 0.4 ± 0.03 ab | 0.3 ± 0.01 a | 0.4 ± 0.02 d | 0.4 ± 0.02 ab | 0.4 ± 0.03 bc | 0.4 ± 0.05 |
| 4A | citric acid | 31.8 ± 3.4 ab | 32.1 ± 2.0 ab | 32.8 ± 2.3 ab | 31.1 ± 3.0 a | 33.2 ± 2.1 ab | 35.1 ± 2.5 b | 32.2 ± 2.3 ab | 30.3 ± 3.1 a | 31.9 ± 4.5 |
| 5A | quinic acid | 0.5 ± 0.03 c | 0.2 ± 0.01 ab | 0.2 ± 0.01 ab | 0.2 ± 0.02 a | 0.2 ± 0.01 ab | 0.3 ± 0.01 b | 0.2 ± 0.01 a | 0.2 ± 0.02 ab | 0.2 ± 0.03 |
| 6A | ascorbic acid | 0.9 ± 0.1 ab | 0.9 ± 0.06 ab | 0.9 ± 0.09 abc | 0.8 ± 0.1 a | 0.9 ± 0.03 abc | 1.0 ± 0.07 c | 0.9 ± 0.05 ab | 1.0 ± 0.08 bc | 0.9 ± 0.1 |
| 7A | galacturonic acid | nd | 0.2 ± 0.03 b | 0.2 ± 0.02 b | 0.2 ± 0.03 a | 0.2 ± 0.08 b | 0.3 ± 0.1 c | 0.3 ± 0.03 c | 0.3 ± 0.04 bc | 0.2 ± 0.1 |
| total | 36.6 ± 0.4 ab | 38.0 ± 3 ab | 37.3 ± 2 ab | 36.2 ± 3 ab | 38.4 ± 2 ab | 40.5 ± 3 b | 37.2 ± 3 ab | 35.1 ± 4 a | 37 ± 6 | |
| Sugars (g/L) | ||||||||||
| 1S | fructose | 36.7 ± 5.0 | 0.1 ± 0.05 a | 0.1 ± 0.02 a | 1.9 ± 0.1 b | 0.2 ± 0.1 a | nd | nd | nd | 0.3 ± 0.1 |
| 2S | glucose | 33.7 ± 3.0 | 0.08 ± 0.08 a | 0.2 ± 0.08 ab | 1.0 ± 0.08 c | 0.2 ± 0.08 b | nd | nd | nd | 0.02 ± 0.05 |
| 3S | sucrose | 7.1 ± 2.2 | 2.8 ± 0.5 c | 0.4 ± 0.3 a | 0.1 ± 0.08 a | 1.1 ± 0.1b | 3.9 ± 0.3 d | 3.9 ± 0.3 d | 3.5 ± 0.5 d | 0.2 ± 0.2 |
| total (g/L) | 77.5 ± 10.0 | 3.0 ± 0.5 cd | 0.6 ± 0.4 a | 2.3 ± 1.4 bc | 1.5 ± 0.2 ab | 3.9 ± 0.3 d | 3.9 ± 0.3 d | 3.5 ± 0.5 d | 0.3 ± 0.1 | |
| Color Analysis | ||||||||||
| yellow (%) | 33.9 ± 0.1 a | 35.0 ± 0.1 bc | 34.2 ± 0.2 a | 36.2 ± 0.2 d | 35.5 ± 0.7 cd | 34.3 ± 0.6 a | 34.2 ± 0.4 a | 34.2 ± 0.4 a | na | |
| red (%) | 33.6 ± 0.1 a | 34.3 ± 0.1 a | 34.0 ± 0.1 a | 35.5 ± 0.2 c | 34.9 ± 0.4 b | 33.6 ± 0.03 a | 33.6 ± 0.04 a | 33.6 ± 0.1 a | na | |
| blue (%) | 32.5 ± 0.1 d | 30.8 ± 0.1 c | 31.8 ± 0.1 cd | 28.4 ± 0.2 a | 29.6 ± 1.0 b | 32.1 ± 0.6 cd | 32.2 ± 0.3 cd | 32.2 ± 0.4 cd | na | |
| color intensity | 7.4 ± 0.03 d | 7.1 ± 0.1 abc | 7.3 ± 0.1 cd | 6.9 ± 0.1 a | 7.0 ± 0.2 ab | 7.2 ± 0.2 bcd | 7.3 ± 0.1 bcd | 7.3 ± 0.1 bcd | na | |
| color tonality | 1.01 ± 0.01 | 1.02 ± 0.01 | 1.01 ± 0.01 | 1.02 ± 0.01 | 1.02 ± 0.01 | 1.02 ± 0.02 | 1.02 ± 0.01 | 1.02 ± 0.01 | na | |
A, organic acid; S, sugar; nd, not detected; na, not calculated; Sc1, S. cerevisiae 1; Sc2, S. cerevisiae 2; Sb, S. bayanus; Td, T. delbrueckii; M1Sc2, M. pulcherrima and S. cerevisiae 2; and M2Sc1, M. fructicola and S. cerevisiae 1. The results are expressed as the average of three biological replicates ± standard deviation. The same letters in each row indicate that there is no significant (p > 0.05) difference between samples.
The averaged beverage was calculated from all of the biological replicates of all of the fermentations.
Statistically significant differences between black currant juice and the average beverage (p < 0.05).
3.2. Ethanol Concentration
The highest ethanol concentration was observed in Sc2 (4.47%, v/v), and the lowest ethanol concentration was in M1Sc2 (3.46%, v/v; Table 1). The ethanol concentration in the Sc2 beverage was significantly higher than those in the beverages from all of the sequential fermentations (3.46–3.79%). A lower ethanol content of sequential fermentations is possibly caused by the lower ethanol production performance of Metschnikowia yeasts. Contreras et al.9 reported significantly lower levels of ethanol from sequential fermentation with S. cerevisiae and five different M. pulcherrima yeast strains than fermentation with S. cerevisiae alone. However, Escribano-Viana et al.32 did not observe almost any difference in the ethanol concentration with sequential fermentations compared to a conventional single yeast fermentation with S. cerevisiae. Our results indicate that Sc2 has a better ethanol production performance than Sc1 in fermenting black currant juice. In addition to the difference between the yeast strains, the ethanol content is often affected to a great extent when another yeast is introduced into the fermentation matrix. Typically, T. delbrueckii is used to reduce the ethanol content in grape wines,33 whereas our study demonstrated that black currant juice fermented with Td had a higher ethanol content (4.07%, v/v) than the Sc1-fermented sample (3.84%, v/v). T. delbrueckii is reportedly highly resistant to stressful environments, such as low pH and temperature.34 This resistance may explain the similar or better fermentation performance of T. delbrueckii in black currant juice in comparison to some S. cerevisiae yeast strains. In addition, T. delbrueckii can be used alone to ferment black currant juice.
3.3. Identification of Phenolic Compounds
A total of 27 phenolic acids, 12 flavonols, 3 proanthocyanidins, and 16 anthocyanins were identified or tentatively identified (Table 2) in the black currant juice and fermented beverages by through comparisons of the retention times, ultraviolet–visible (UV–vis) spectra, and mass spectra to the reference compounds and the results reported in the literature.3,20,35−37
Table 2. Identification of Hydroxycinnamic Acid Derivatives, Flavonols, Flavan-3-ols, and Anthocyanins in Fermented Black Currant Beverages.
| MS (m/z)+ |
MS2 | MS (m/z)– |
MS2 | exact mass (molecule) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| compound number | tentative identificationa | λmax (nm) | [M + H]+ exact mass/calculated mass | [M + H]+ (error ppm) | [M + H]+ | [M – H]− exact mass/calculated mass | [M – H]+ (error, ppm) | [M – H]+ | measurement/calculated | M (error, ppm) | molecular formula | identification | ref |
| 1 | citric acid methyl ester | 195, 294 | nd | nd | nd | 205.0343/205.0354 | 5.0 | 191.0176, 111.0075, 143.0332 | 206.0416/206.0427 | –5.3 | C7H10O7 | UV, QTOF | (20) |
| Hydroxycinnamic Acids | |||||||||||||
| 2 | 4-O-caffeoylglucoside | 215, 328 | nd | nd | nd | 341.0846/341.0878 | 9.3 | 161.0230, 133.0280 | 342.0919/342.0951 | –9.4 | C15H18O9 | UV, QTOF | (20) |
| 3 | p-coumaric acid O-glucoside | 208, 294 | nd | nd | nd | 325.0900/325.0929 | 9 | 163.0386, 119.0486 | 326.0973/326.1002 | –8.9 | C15H18O8 | UV, QTOF | (20) |
| 4 | (E)-caffeoylglucose | 216, 328 | nd | nd | nd | 341.0845/341.0819 | 7.6 | 161.0226, 133.0278, 683.1716 | 342.0918/342.0892 | 7.6 | C15H18O9 | UV, QTOF | (20) |
| 5 | p-coumaroylquinic acid | 208, 311 | 339.1075/339.1074 | –0.1 | 147.0446, 119.0486 | 337.0890/337.0870 | –5.8 | 163.0382 | 338.0963/338.0943 | 5.9 | C16H16O8 | UV, QTOF | (20) |
| 6 | (Z)-p-coumaric acid O-glucoside | 210, 311 | nd | nd | nd | 325.0924/325.0929 | 1.5 | 145.0291 | 326.0997/326.1002 | –1.5 | C15H18O9 | UV, QTOF | (20) |
| 7 | caffeic acid | 217, 323 | 181.0512/181.0495 | –9.3 | 163.0405 | 179.0339/179.0350 | 6 | 161.0229, 339.0679 | 180.0412/180.0423 | –6.1 | C9H8O4 | UV, QTOF | (7,20) |
| 8 | ferulic acid hexoside | 214, 327 | nd | nd | nd | 355.0988 | nd | 193.0481, 175.0378 | 356.1061 | nd | nd | UV, QTOF | (7.20) |
| 9 | p-coumaric acid | 207, 309 | nd | nd | nd | nd | nd | nd | nd | nd | nd | STD, HPLC | (7) |
| 10 | 2-(E)-caffeoyloxymethylene-4-β-d-glucopyranosyloxy-2-(Z)-butenenitrile [(E)-caffeoyloxymethylene glucosyloxybutenenitrile] | 213, 330 | nd | nd | nd | 436.1191/436.1190 | nd | nd | 437.1264/437.1263 | 0.2 | UV, QTOF | (20) | |
| 11 | 2-(E)-p-coumaroyloxymethylene-4-β-d-glucopyranosyloxy-2-(Z)-butenenitrile [(E)-coumaroyloxy methyleneglucosyloxybutenenitrile] | 210, 313 | 422.1445/422.1446 | 0.1 | 114.0549, 147.0436 | 420.1257/420.1255 | –0.4 | 163.0385, 119.0492 | 421.133/421.1328 | 0.5 | C20H23NO9 | UV, QTOF | (20) |
| 12 | 2-(Z)-p-coumaroyloxymethylene-4-β-d-glucopyranosyloxy-2-(Z)-butenenitrile [(Z)-coumaroyloxy methyleneglucosyloxybutenenitrile] | 216, 310 | 422.144/422.1446 | 0.5 | 114.0548 | 420.1256/420.1300 | nd | 163.0389 | 421.1329/421.1373 | –10.4 | C20H23NO9 | UV, QTOF | (20) |
| 13 | 2-(E)-feruloyloxymethylene-4-β-d-glucopyranosyloxy-2-(Z)-butenenitrile [(E)-feruloyloxymethylene glucosyloxybutenenitrile] | 218, 327 | 452.1543/452.1551 | 0.8 | 114.0539, 177.0542 | 450.1355/450.1397 | nd | 193.0485 | 451.1428/451.1470 | –9.3 | C21H25NO10 | UV, QTOF | (20) |
| 14 | 2-(E)-p-coumaroyloxymethylenesyloxy-2-(Z)-butenenitrile [(E)-coumaroyloxy methylenesyloxybutenenitrile] | 217, 313 | nd | nd | nd | 258.0756/258.0771 | nd | 163.0390, 184.0755, 214.0859 | 259.0829/259.0844 | –5.8 | C14H13NO4 | UV, QTOF | (20) |
| 15 | 2-(Z)-p-coumaroyloxymethylenesyloxy-2-(Z)-butenenitrile [(Z)-coumaroyloxy methylenesyloxybutenenitrile] | 219, 310 | nd | nd | nd | 258.0758/258.0771 | nd | 163.0390, 214.0861 | 259.0831/259.0844 | –5.0 | C14H13NO4 | UV, QTOF | (20) |
| Flavonols | |||||||||||||
| 16 | myricetin-3-O-rutinoside | 210, 356 | 627.1587/627.1556 | –5 | 319.0462, 481.0996 | 625.1320/625.1351 | 5 | nd | 626.1393/626.1424 | –5.0 | C27H30O17 | UV, QTOF | (7,20) |
| 17 | myricetin-3-O-glucoside | 210, 355 | 481.0977/481.0996 | –3.9 | 319.0461 | nd | nd | nd | 480.0904/480.0923 | –4.0 | C21H20O13 | UV, QTOF | (7,20) |
| 18 | myricetin-3-O-arabinoside | 216, 355 | 451.0891/451.087 | –4.4 | 319.0458 | nd | nd | nd | 450.0818/450.0797 | 4.7 | C20H18O12 | UV, QTOF | (20) |
| 19 | myricetin-3-O-(6″-malonyl)-galactoside | 208, 254 | 567.0997/567.0981 | –2.9 | 319.046 | 565.0824/565.0835 | 2 | 521.0924 | 564.0751/564.0762 | –2.0 | UV, QTOF | (20) | |
| 20 | quercetin-3-O-rutinoside | 205, 352 | 611.1622/611.1607 | –2.6 | 465.1037, 303.0505 | nd | nd | nd | 610.1549/610.1534 | 2.5 | C27H31O16 | UV, QTOF, STD | (20) |
| 21 | quercetin-3-O-glucoside | 205, 352 | 465.1032/465.1028 | –1 | 417.1369, 303.0500 | nd | nd | nd | 464.0959/464.0955 | 0.9 | C21H20O12 | UV, QTOF, STD | (20) |
| 22 | kaempferol-3-O-rutinoside | 208, 350 | 595.165/595.1657 | 1.3 | 449.1075, 287.0536 | 593.1438/593.1453 | 2.5 | 284.0294 | 594.1511/594.1526 | –2.5 | C27H30O15 | UV, QTOF | (20) |
| 23 | quercetin-3-O-(6″-malonyl)-glucoside | 218, 352 | 551.1026/551.1031 | 1 | 303.049 | nd | nd | nd | 550.0953/550.0958 | –0.9 | C24H22O15 | UV, QTOF | (20) |
| 24 | kaempferol-3-O-galactoside | 210, 343 | 449.1076/449.1078 | 0.6 | 287.0549 | nd | nd | nd | 448.1003/448.1005 | –0.4 | C21H20O11 | UV, QTOF | (20) |
| 25 | myricetin | 219, 371 | nd | nd | nd | nd | nd | nd | UV | (20) | |||
| 26 | quercetin | 220, 368 | 303.0505/303.0499 | –1.8 | nd | nd | nd | nd | 302.0432/302.0426 | –2.8 | C15H10O7 | UV, QTOF | (20) |
| Flavan-3-ols | |||||||||||||
| 27 | epi-gallocatechin | 210, 287 | 307.0813/307.0812 | –0.1 | nd | 305.0644/305.0666 | 7.6 | 611.1325 | 306.0717/306.0739 | –7.2 | C15H14O7 | UV, QTOF | (1) |
| 28 | catechin | 202, 283 | nd | nd | nd | 289.0682/289.0718 | 12.3 | 465.0979 | 290.0755/290.0791 | –12.4 | C15H14O6 | UV, QTOF | (1) |
| 29 | epi-catechin | 204, 279 | 291.0871/291.0863 | –0.7 | nd | nd | nd | nd | 290.0798/290.079 | 2.8 | C15H14O6 | UV, QTOF | (1) |
| Anthocyanins | |||||||||||||
| 30 | delphinidin-3-O-glucoside | 277, 523 | 465.1051/465.1028 | nd | nd | nd | nd | nd | 464.0978/464.0955 | 5.0 | C21H21O12 | UV, QTOF | (20,36,37) |
| 31 | delphinidin-3-O-rutinoside | 277, 524 | 611.1599/611.1607 | 1.3 | nd | nd | nd | nd | 610.1526/610.1534 | –1.3 | C27H31O16 | UV, QTOF | (20) |
| 32 | cyanidin-3-O-glucoside | 279, 514 | 449.1083/449.1078 | –1.1 | nd | nd | nd | nd | 448.1010/448.1005 | 1.1 | C21H21O11 | UV, QTOF | (20,36,37) |
| 33 | cyanidin-3-O-rutinoside | 280, 516 | 595.166/595.1657 | –0.5 | nd | nd | nd | nd | 594.1587/594.1584 | 0.5 | C27H31O15 | UV, QTOF | (20,36,37) |
| 34 | petunidine-3-O-rutinoside | 526, 277 | 625.1758/625.1763 | 0.9 | 433.1122 | nd | nd | nd | 624.1685/624.1690 | –0.8 | C28H33O16 | UV, QTOF | (35) |
| 35 | peonidin-3-O-rutinoside | 273, 515 | 609.1811/609.1814 | 0.5 | nd | nd | nd | nd | 608.1738/608.1741 | –0.5 | C28H33O15 | UV, QTOF | (35) |
| 36 | delphinidin 3-O-(6″-coumaroyl)-glucoside | 281, 528 | 611.1368/611.1395 | 4.5 | 303.8837 | nd | nd | nd | 610.1295/610.1322 | –4.4 | C30H27O14 | UV, QTOF | (7) |
| delphinidin | 267, 505 | nd | nd | nd | nd | nd | nd | nd | nd | C15H11O7 | UV | (7) | |
| 37 | cyanidin 3-O-(6″-coumaroyl)-glucoside | 281, 523 | 595.143/595.1446 | 2.8 | 287.0542 | nd | nd | nd | 594.1357/594.1373 | –2.7 | C28H33O15 | UV, QTOF | (7) |
| 38 | cyanidin | 278, 503 | nd | nd | nd | nd | nd | nd | nd | nd | C15H11O6 | UV | (7) |
| Pyranoanthocyanins | |||||||||||||
| 39 | cyanidin-3-O-rutinoside pyruvic acid | nd | 663.1551/663.1556 | 0.7 | 595.1648 | nd | nd | nd | 662.1478/662.1483 | –0.8 | C30H31O17 | QTOF | (37) |
| 40 | cyanidin-3-O-rutinoside acetaldehyde | nd | 619.1653/619.1657 | 0.7 | 595.1584 473.1092 | nd | nd | nd | 618.158/618.1584 | –0.6 | QTOF | (37) | |
Phenolic compounds were identified using HPLC–DAD–ESI–QTOF by comparing the reference standards and previous literature. nd = not detected.
In the phenolic acid group, derivatives of caffeic, p-coumaric, and ferulic acids were detected in addition to free caffeic acid and p-coumaric acid. Four phenolic acids containing a nitrile group were also detected in the black currant juice: (E)-caffeoyloxymethyleneglucosyloxybutenenitrile, (E)-coumaroyloxymethyleneglucosyloxybutenenitrile, (Z)-coumaroyloxymethyleneglucosyloxybutenenitrile, and (E)-feruloyloxymethyleneglucosyloxybutenenitrile (compounds 10–13 in Table 2), which have been identified before by Lu et al. and Mäkilä et al.20,38 In comparison to the black currant juice, two new phenolic acid peaks were detected only in the fermented samples, namely, (E)-coumaroyloxymethyleneoxybutenenitrile and (Z)-coumaroyloxymethyleneoxybutenenitrile (compounds 14 and 15 in Table 2). They were tentatively identified on the basis of the mass spectra as aglycones of compounds 11 and 12, respectively. The odd-numbered nominal mass [M] of m/z 259 indicated the presence of odd-numbered nitrogen atoms in the molecules. The fragmentation pattern under both ionization modes (m/z+ 96, 114, 147, and 242 [M + H – H2O]; m/z– 117 and 163 [M – H – coumaric acid]) were similar to those of compounds 11 and 12. These compounds are most likely formed through the cleavage of glucose by yeast glucosidases.
Flavonols were identified as glycosides of myricetin, quercetin, and kaempferol, and anthocyanins were identified as glycosides of delphinidin, cyanidin, and peonidin. Two pyranoanthocyanins, cyanidin-3-O-rutinoside-pyruvic acid and cyanidin-3-O-rutinoside-acetaldehyde (compounds 39 and 40 in Table 2), were detected and tentatively identified in fermented beverages with a quadrupole time-of-flight (QTOF) mass spectrometer. The parent ion masses were [M + H]+ 663 and 619, respectively. The daughter ions of the peaks were both in m/z+ 595, indicating that both compounds were cyanidin-3-O-rutinoside derivatives. Compound 39 was tentatively identified as cyanidin-3-O-rutinoside-pyruvic acid ([M + H + pyruvic acid]+ 595 + 68), and compound 40 was cyanidin-3-O-rutinoside-acetaldehyde ([M + H + acetaldehyde]+ 595 + 24). Pyranoanthocyanins were detected in the trace amounts in all fermented beverages but were absent from the black currant juice. The formation of a low amount of pyranoanthocyanins during black currant fermentation could be attributed to low concentrations of nutrients, such as sugars and nitrogen, which limited the formation of pyruvic acid and acetaldehyde during the early stage of fermentation. The sugar content of the grape musts ranges between 150 and 250 g/L, whereas in the black currant juice used in this study, it was only 78 g/L. In a nutrient-rich environment, pyruvic acid is produced in excess and excreted from yeast cells into the fermentation medium, which leads to a high formation rate of pyranoanthocyanins in wines. Additionally, the pH and temperature affect the formation rate of pyranoanthocyanins.39 The pH of grapes must can be as high as 3.6,40 whereas the black currant juice pH is typically below 3 (in this study, it was 2.96).41
3.4. Comparison between Chemical Compositions of Black Currant Juice and Fermented Beverages
The independent sample t test was used to compare the phenolic compositions of black currant juice and the averaged value of all fermented black currant beverages (Table 3). Generally, the phenolic profiles differed between the fermented beverages and the black currant juice.
Table 3. Contents of Hydroxycinnamic Acids, Flavan-3-ols, Flavonols, Anthocyanins, and Citric Acid Methyl Esters in Black Currant Juice and Fermented Beveragesa.
| number | compound name | juice | Sc1 | Sc2 | Sb | Td | M1Sc1 | M1Sc2 | M2Sc1 | averaged beverageb |
|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxycinnamic Acids (mg/L) | ||||||||||
| 1 | (E)-caffeoylglucose | 19.4 ± 6 ab | 19.3 ± 2.4 ab | 18.7 ± 1.2 a | 19.2 ± 3.0 ab | 19.0 ± 2.1 a | 21.0 ± 1.7 ab | 23.0 ± 1.8 b | 20.1 ± 1.6 ab | 20.0 ± 2.4 |
| 2 | p-coumaric acid O-glucoside | 1.3 ± 0.5 a | 1.5 ± 0.2 ab | 1.8 ± 0.2 b | 1.9 ± 0.3 b | 1.7 ± 0.3 b | 1.6 ± 0.1 ab | 1.6 ± 0.1 ab | 1.7 ± 0.2 b | 1.7 ± 0.2 |
| 3 | 1-O-caffeoylglucose | 1.8 ± 0.4 abc | 1.8 ± 0. 2 abc | 1.7 ± 0.3 a | 1.7 ± 0.2 ab | 1.7 ± 0.2 a | 2.1 ± 0.2 c | 1.8 ± 0.1 abc | 2.1 ± 0.2 bc | 1.8 ± 0.2 |
| 4 | coumaroylquinic acid | 1.9 ± 0.3 a | 1.8 ± 0.2 a | 1.9 ± 0.2 a | 1.9 ± 0.2 a | 1.8 ± 0.1 a | 2.0 ± 0.1 ab | 2.3 ± 0.1 b | 2.0 ± 0.1 a | 2.0 ± 0.2 |
| 5 | 4-O-coumaroylglucose | 10.9 ± 1.0 a | 10.0 ± 1.2 a | 10.2 ± 0.5 a | 10.2 ± 1.1 a | 10.0 ± 1.0 a | 11.0 ± 0.6 ab | 12.4 ± 0.7 b | 10.9 ± 0.5 a | 10.7 ± 0.1 |
| 6 | ferulic acid hexoside | 1.9 ± 0.4 ab | 1.8 ± 0.2 ab | 1.9 ± 0.2 ab | 1.9 ± 0.3 ab | 1.7 ± 0.2 a | 2.0 ± 0.1 b | 2.0 ± 0.2 b | 1.9 ± 0.2 ab | 1.9 ± 0.2 |
| 7 | (Z)-p-coumaroylglucose | 0.7 ± 0.3 a | 0.9 ± 0.2 ab | 1.1 ± 0.2 bc | 1.4 ± 0.3 c | 1.1 ± 0.3 bc | 0.9 ± 0.09 ab | 1.0 ± 0.2 abc | 1.1 ± 0.5 bc | 1.1 ± 0.3c |
| 8 | ferulic acid hexoside | 3.0 ± 0.4 a | 5.2 ± 0.6 c | 5.4 ± 0.3 c | 3.7 ± 0.4 b | 3.5 ± 0.3 ab | 7.2 ± 0.3 e | 6.3 ± 0.8 d | 7.0 ± 0.4 e | 5.5 ± 1.4c |
| 9 | p-coumaric acid | 1.2 ± 0.1 a | 1.5 ± 0.3 a | 4.6 ± 0.4 e | 2.1 ± 0.2 b | 2.6 ± 0.3 c | 4.5 ± 0.2 e | 4.2 ± 0.5 e | 3.1 ± 0.4 d | 3.3 ± 1.2c |
| 10 | (E)-caffeoyloxymethylen eglucosyloxybutanenitrile | 3.0 ± 0.2 | nd | nd | nd | nd | nd | nd | nd | ndc |
| 11 | (E)-p-coumaroyloxymethylene glucosyloxybutenenitrile | 7.3 ± 0.5 d | 0.5 ± 0.1 a | 0.5 ± 0.2 a | 2.0 ± 0.6 c | 0.9 ± 0.3 a | 1.6 ± 0.1 b | 0.5 ± 0.07 a | 0.5 ± 0.1 a | 0.9 ± 0.6c |
| 12 | (Z)-p-coumaroyloxymethylene glucosyloxybutenenitrile | 1.4 ± 0.2 d | 0.5 ± 0.01 a | 0.5 ± 0.06 ab | 0.9 ± 0.2 c | 0.9 ± 0.09 b | 0.6 ± 0.02 ab | 0.05 ± 0.03 a | 0.6 ± 0.1 ab | 0.6 ± 0.1c |
| 13 | (E)-feruloyloxymethylene glucosyloxybutanenitrile | 5.1 ± 0.4 c | 0.3 ± 0.01 a | 0.3 ± 0.06 a | 0.8 ± 0.2 b | 0.4 ± 0.1 a | 0.7 ± 0.05 b | 0.3 ± 0.05 a | 0.3 ± 0.05 a | 0.4 ± 0.2c |
| 14 | (E)-p-coumaroyloxymethylene oxybutenenitrile | nd | 3.5 ± 0.4 b | 3.4 ± 0.4 b | 2.9 ± 0.3 ab | 2.7 ± 0.5 a | 5.3 ± 0.3 d | 4.8 ± 0.7 cd | 4.4 ± 0.3 c | 3.8 ± 1.0c |
| 15 | (Z)-p-coumaroyloxymethylene oxybutenenitrile | nd | 0.44 ± 0.08 a | 0.5 ± 0.07 a | 0.5 ± 0.07 a | 0.4 ± 0.09 a | 0.7 ± 0.04 b | 0.7 ± 0.04 b | 0.6 ± 0.1 b | 0.5 ± 0.1c |
| total | 59 ± 9 cd | 54 ± 3 a | 57 ± 3 abc | 55 ± 7 ab | 53 ± 4 a | 68 ± 4 d | 67 ± 5 d | 63 ± 3 bcd | 59.5 ± 7c | |
| Flavan-3-ols (mg/L) | ||||||||||
| 16 | epi-gallocatechin | 11 ± 3 ab | 10 ± 1 a | 11 ± 1 ab | 12 ± 2 b | 11 ± 1 ab | 13 ± 1 b | 13 ± 1 b | 13 ± 1 b | 12 ± 2 |
| 17 | catechin | 35 ± 10 | 36 ± 4 | 38 ± 2 | 39 ± 5 | 38 ± 4 | 41 ± 3 | 41 ± 3 | 40 ± 4 | 39 ± 4 |
| 18 | PB-2 | 4.8 ± 0.2 | 4 ± 0.8 | 4.5 ± 1.3 | 4.9 ± 0.8 | 3.9 ± 0.1 | 4.1 ± 0.1 | 4.0 ± 0.3 | 4.3 ± 0.6 | 4.0 ± 1 |
| total | 50 ± 14 | 50 ± 6 | 53 ± 4 | 57 ± 7 | 53 ± 6 | 58 ± 3 | 57 ± 4 | 57 ± 5 | 55 ± 6 | |
| Flavonols (mg/L) | ||||||||||
| 19 | myricetin rutinoside | 4.8 ± 2.1 a | 6.8 ± 0.5 b | 7.5 ± 0.3 b | 7.4 ± 1.1 b | 7.2 ± 1.0 b | 7.2 ± 0.5 b | 7.2 ± 0.7 b | 7.2 ± 0.9 b | 7 ± 1c |
| 20 | myricetin glucoside | 24.4 ± 4.2 | 24.7 ± 0.6 a | 27.3 ± 1.7 a | 26.9 ± 2.8 a | 24.6 ± 2.3 a | 33.0 ± 1.5 b | 28.2 ± 2.5 a | 34.5 ± 3.4 b | 28 ± 4c |
| 21 | myricetin arabinoside | 0.7 ± 0.04 c | 0.5 ± 0.03 ab | 0.6 ± 0.1 ab | 0.6 ± 0.1 ab | 0.5 ± 0.1 ab | 0.5 ± 0.03 bc | 0.5 ± 0.04 ab | 0.6 ± 0.1 ab | 1 ± 0.1c |
| 22 | myricetin malonylgalactoside | 8.2 ± 0.7 a | 7.6 ± 0.2 a | 8.1 ± 0.8 a | 8.0 ± 0.8 a | 7.5 ± 0.9 a | 10.7 ± 0.6 bc | 9.6 ± 0.9 b | 11.1 ± 1.1 c | 9 ± 2 |
| 23 | quercetin rutinoside | 10.0 ± 3.0 a | 10.3 ± 0.5 ab | 11.3 ± 0.5 ab | 11.7 ± 1.4 ab | 10.9 ± 1.3 ab | 11.7 ± 0.7 ab | 11.8 ± 1.0 ab | 12.0 ± 1.0 b | 11 ± 1 |
| 24 | quercetin glucoside | 18.9 ± 0.5 a | 17.3 ± 0.4 a | 18.7 ± 1.3 a | 18.4 ± 1.9 a | 16.9 ± 1.0 a | 23.0 ± 1.0 b | 22.2 ± 1.6 b | 23.9 ± 1.8 b | 20 ± 3c |
| 25 | kaempferol rutinoside | 6.4 ± 0.3 ab | 5.4 ± 0.2 a | 6.1 ± 0.4 a | 5.9 ± 0.6 a | 5.4 ± 0.6 a | 7.5 ± 0.5 c | 7.2 ± 0.6 bc | 7.7 ± 0.8 c | 6 ± 1 |
| 26 | quercetin malonylglucoside | 0.7 ± 0.03 ab | 0.9 ± 0.1 ab | 0.9 ± 0.3 ab | 0.5 ± 0.1 a | 1.0 ± 0.1 ab | 0.9 ± 0.2 ab | 0.7 ± 0.1 ab | 0.9 ± 0.1 ab | 1 ± 0.4 |
| 27 | kaempferol galactoside | 4.5 ± 0.5 b | 3.7 ± 0.1 a | 3.8 ± 0.3 a | 3.9 ± 0.4 a | 3.6 ± 0.2 a | 5.5 ± 0.3 c | 4.8 ± 0.3 b | 5.6 ± 0.4 c | 4 ± 1 |
| 28 | myricetin | 1.5 ± 0.1 b | 1.3 ± 0.1 ab | 1.3 ± 0.2 ab | 1.3 ± 0.2 ab | 1.2 ± 0.1 a | 2.0 ± 0.1 c | 1.3 ± 0.2 ab | 2.3 ± 0.3 d | 2 ± 0.4 |
| 29 | quercetin | 0.8 ± 0.1 b | 0.5 ± 0.03 a | 0.6 ± 0.1 a | 0.5 ± 0.1 a | 0.4 ± 0.1 a | 1.2 ± 0.07 c | 0.8 ± 0.1 b | 1.2 ± 0.1 c | 1 ± 0.3 |
| total | 81 ± 10 a | 79 ± 2 a | 86 ± 5 ab | 85 ± 9 ab | 79 ± 7 a | 103 ± 5 cd | 94 ± 8 bc | 107 ± 10 d | 90 ± 12 | |
| Anthocyanins (mg/L) | ||||||||||
| 30 | delphinidin glucoside | 138 ± 4 d | 87 ± 15 b | 93 ± 13 b | 61 ± 19 a | 94 ± 8 b | 125 ± 28 cd | 111 ± 17 bc | 132 ± 16 cd | 95 ± 34 |
| 31 | delphinidin rutinoside | 455 ± 12 d | 323 ± 26 b | 345 ± 47 b | 221 ± 31 a | 348 ± 57 bc | 422 ± 53 cd | 396 ± 62 bcd | 433 ± 60 cd | 335 ± 110 |
| 32 | cyanidin glucoside | 77 ± 2 e | 40 ± 3 ab | 43 ± 6 ab | 32 ± 4 a | 48 ± 9 b | 61 ± 8 cd | 51 ± 8 bc | 64 ± 10 d | 46 ± 16 |
| 33 | cyanidin rutinoside | 463 ± 12 d | 322 ± 25 b | 345 ± 49 b | 220 ± 30 a | 346 ± 58 bc | 430 ± 54 cd | 393 ± 62 bcd | 445 ± 62 d | 336 ± 113 |
| 34 | petunidin rutinoside | 12 ± 0.3 d | 9 ± 0.7 abc | 9 ± 1 ab | 6 ± 0.8 a | 9 ± 1 bc | 11 ± 1 cd | 10 ± 2 bcd | 11 ± 1 cd | 9 ± 3 |
| 35 | peonidin rutinoside | 8.9 ± 0.2 c | 5.6 ± 1.9 ab | 12 ± 2 ab | 4.4 ± 0.6 a | 6.7 ± 1.0 abc | 8.2 ± 1.0 bc | 6.5 ± 3 ab | 8.3 ± 1.1 bc | 6 ± 3 |
| 36 | delphinidin 3-O-(6-p-coumaroyl) glucoside | 3.7 ± 0.1 e | 2.4 ± 0.2 b | 3 ± 0.4 b | 1.6 ± 0.2 a | 2.5 ± 0.5 bc | 3.0 ± 0.4 cd | 2.8 ± 0.4 bcd | 3.2 ± 0.5 de | 2 ± 1 |
| 37 | delphinidin (aglycone) | nd | 0.9 ± 0.1 b | nd | 0.2 ± 0.05 a | 0.2 ± 0.1 b | 0.1 ± 0.06 bc | nd | 0.3 ± 0.06 c | 0.3 ± 0.03c |
| 38 | cyanidin 3-O-(6-p-coumaroyl) glucoside | 1.6 ± 0.4 d | 1.0 ± 0.1 b | 1.0 ± 0.2 b | 0.1 ± 0.2 b | 0.7 ± 0.9 a | 1.2 ± 0.2 bc | 1.2 ± 0.2 c | 1.2 ± 0.14 bc | 1 ± 0.3 |
| 39 | cyanidin (aglycone) | 0.07 ± 0.01 a | 0.9 ± 0.1 d | 0.1 ± 0.02 a | 0.3 ± 0.1 bc | 0.3 ± 0.1 bc | 0.2 ± 0.07 ab | nd | 0.4 ± 0.05 c | 0.3 ± 0.01c |
| total | 1159 ± 30 c | 905 ± 207 abc | 873 ± 81 ab | 637 ± 144 a | 821 ± 159 ab | 1153 ± 113 c | 765 ± 272 ab | 989 ± 145 bc | 830 ± 280 | |
| 40 | citric acid methyl esters (mg/L) | 67 ± 2 ab | 62 ± 8 a | 67 ± 4 ab | 66 ± 5 ab | 63 ± 2 ab | 91 ± 2 c | 71 ± 4 b | 89 ± 9 c | 73 ± 12c |
BC, black currant; Sc1, S. cerevisiae 1; Sc2, S. cerevisiae 2; Sb, S. bayanus; Td, T. delbrueckii; M1Sc1 M. pulcherrima and S. cerevisiae 1; M1Sc2 M. pulcherrima and S. cerevisiae 2; and M2Sc1 M. fructicola and S. cerevisiae 1. The results are expressed as the average of three biological replicates ± standard deviation. The contents of phenolic acids are expressed as equivalents of p-coumaric acid (310 nm) or chlorogenic acid (320 nm); the contents of flavan-3-ols are expressed as equivalents of (+)-catechin; the contents of flavonols are expressed as equivalents of quercetin-3-O-glucoside; and the contents of anthocyanins are expressed as equivalents of cyanidin-3-O-glucoside. The same letters in each row indicate that there is no significant (p > 0.05) difference between samples.
Averaged beverage was calculated from all of the biological replicates of fermentations.
Statistically significant difference between black currant juice and averaged beverage (p < 0.05).
Seven organic acids and three sugars were detected in the juice and fermented beverages (Table 1). Not surprisingly, the contents of all of the sugars were significantly lower in the fermented beverages than in the black currant juice. Interestingly, the total organic acids differed less than 1% between the juice and average beverage as a result of the fact that citric acid as the primary acid remained unaffected. Citric acid accounted for up to 87% of the total organic acid contents. Methyl esters of citric acid have been detected before in black currants20 and were also detected in the juice and fermented beverages in our study. The average concentrations of citric acid methyl esters were statistically significantly higher in fermented black currant beverages (73 mg/L) than the corresponding values in the black currant juice (67 mg/L). In contrast, the fermentations primarily decreased the concentrations of malic, shikimic, and quinic acids. Succinic and galacturonic acids were not present in the black currant juice but appeared in the all of the fermentations (Table 3). Yeasts produce succinic acid during alcohol fermentation through a few different metabolic pathways,42 and galacturonic acid is formed as a degradation product of pectin by pectinases.
Fermentation decreased the total concentration of hydroxycinnamic acids (by 6.7%) and the total content of anthocyanins (by 28.4%) and increased the total concentration amounts of flavonols (11.7%) and flavan-3-ols (8.8%). The contents of all of the hydroxycinnamic acids containing a nitrile moiety decreased significantly during the fermentations compared to the black currant juice. In addition, the contents of Z-p-coumaroylglucose, ferulic acid hexoside, and p-coumaric acid were significantly higher in the fermented beverages than in the juice. Czyzowska and Pogorzelski22 reported that the hydroxycinnamic acid contents decreased by approximately half during fermentation. In general, all monomeric anthocyanin contents decreased during the fermentations. The most highly affected anthocyanins were the glucosides of cyanidin, delphinidin, and cyanidin 3-O-(6-p-coumaroyl) glucoside. Their contents decreased by 40.8, 37.5, and 34.7%, respectively. Simultaneously, the concentrations of delphinidin and cyanidin aglycones increased during the fermentations. Anthocyanins have an important role in consumer acceptance of food products because their color is one of the first things consumers can perceive in foods. Czyżowska and Pogorzelski21 reported a higher loss of anthocyanins (96%) after a wine yeast fermentation of black currant juice treated with pectinase compared to our results.
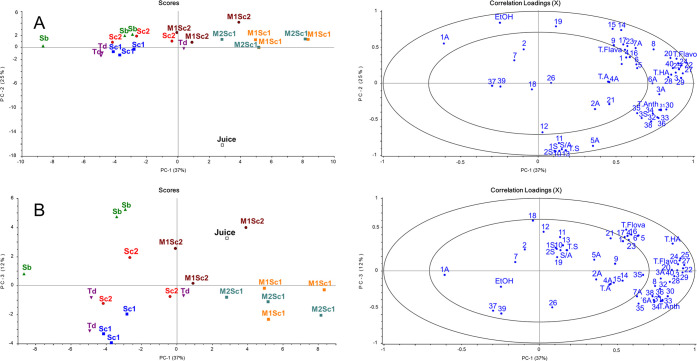
A PCA model constructed with all of the samples (n = 21; biological replicates shown separately) and all of the chemical variables (n = 57; first three PCs explain 74% of the total variance) clearly shows that black currant juice is clearly separated from the fermentation samples on the PC2 (panels A and B of Figure 2). Not surprisingly, the ethanol and total sugar contents correlate negatively with one another. However, most of the organic acids and the total organic acid content correlate more positively with fermented beverages than with black currant juice. The nitrile-containing hydroxycinnamic acids (variables 10–15), certain anthocyanins (e.g., rutinosides of delphinidin and cyanidin), and the majority of sugars correlate positively with black currant juice. The aglycones of the nitrile-containing hydroxycinnamic acids, ethanol, and myricetin rutinoside correlated negatively with the black currant juice.
Figure 2.
PCA based on 57 chemical variables in black currant juice and fermented black currant beverages using the following inoculation schemes: (black □) black currant juice, (blue ■) S. cerevisiae 1, (red ●) S. cerevisiae 2, (green ▲) S. bayanus, (purple ▼) T. delbrueckii, (orange ■) M. pulcherrima + S. cerevisiae 1, (cyan ■) M. fructicola + S. cerevisiae 1, and (maroon ●) M. pulcherrima + S. cerevisiae 2 for (A) PC1 versus PC2, and (B) PC1 versus PC3. Numbers with A (acid) or S (sugar) refer to Table 1, and the numbers of variables refer to Table 3. T.HA, total hydroxycinnamic acids; T.Flava, total flavan-3-ols; T.Flavo, total flavonols; T.Anth, total anthocyanins; T.A, total organic acids; T.S, total sugars; and S/A, sugar/acid ratio.
3.5. Comparison of Chemical Composition of Beverages Fermented with Different Yeast Strains
3.5.1. Organic Acids and Residual Sugars
Citric acid was the primary organic acid in the black currant beverages. Its concentrations varied between 30.3 g/L (M2Sc1) and 35.2 g/L (M1Sc1). The concentrations of citric acid increased moderately in all of the other fermentations, except Sb and M2Sc1.
The highest concentration of succinic acid was found in the Sb beverage (930 mg/L); the second highest concentration of succinic acid was found in the Td beverage (770 mg/L); and the lowest concentrations were observed in the sequentially fermented beverages. Our results are concurrent with Eglinton et al.,43 who reported that S. bayanus produced more succinic acid than S. cerevisiae during fermentation. In addition, high production rates of succinic acid by T. delbrueckii have been reported before.30,33 Canonico et al.44 reported increases in the succinic acid concentration when a beverage was fermented with M. pulcherrima and S. cerevisiae, and Liu et al.45 reported similar succinic acid levels in sequential fermentation with M. fructicola and S. cerevisiae. In our study, the concentrations of succinic acid in the sequentially fermented beverages were significantly lower than they were in the Sc1 and Sc2 beverages. According to Rubico and McDaniel,46 the taste properties of succinic acid are sour and bitter at a concentration of 680 mg/L. In the present study, the concentrations of succinic acid in the fermented beverages were above the reported threshold, indicating possible effects on the sensory properties of the beverages.
The highest galacturonic acid concentrations were in the sequential fermentations (280–310 mg/L), and the lowest galacturonic acid concentration was in Sb (160 mg/L). Belda et al.16 reported that both M. pulcherrima and M. fructicola have high polygalacturonase activity, and the use of M. pulcherrima with S. cerevisiae was beneficial for the sensory and technological properties of wine products as a result of the release of important sensory active compounds by pectin degradation.
Clear differences were observed in the contents of residual sugars between the yeasts studied here (Table 1). Sc1 had the lowest amounts of residual glucose (0.08 g/L) and fructose (0.1 g/L), whereas Sb had the highest amount (1.0 and 1.9 g/L, respectively) but the lowest amount of sucrose (0.1 g/L). Sc2 also had a low amount of sucrose left (0.4 g/L), whereas Sc1 had a higher amount (2.8 g/L). Interestingly, sequential fermentations had the highest amount of sucrose left after fermentation (3.5–3.9 g/L), corresponding to approximately half of the initial sucrose concentration and 10 times the level in Sc2. Additionally, no glucose or fructose was detected after the sequential fermentations. Differences in the use of sugar may result from different gene expressions across the yeast strains and species. Generally, yeasts preferably use hexose monosaccharides, such as glucose and fructose, because these sugars enter the glycolytic pathway directly. To degrade sucrose, yeasts have to first produce the invertase enzyme. High concentrations of glucose suppress the expression of the genes encoding the invertase enzyme. Therefore, when glucose is present at high levels, the production of invertase is inhibited by yeast.47
3.5.2. Phenolic Acids, Flavonols, and Anthocyanins
The phenolic acid contents in the fermented samples varied between 53 and 67 mg/L (Table 3). In general, the total phenolic acid contents were significantly lower in all of the single yeast fermentations compared to the sequential fermentations.
Six phenolic acids containing nitrile groups (compounds 10–15 in Table 2) were detected in the black currant juice and beverages. The contents of nitrile compounds decreased 1.5–19-fold (Table 3) during the fermentations, and one of the nitrile compounds was detected only in trace amounts. Two new nitrile compounds were detected in the fermented black currant beverages, which were tentatively identified as aglycones of the compounds (E)-coumaroyloxymethyleneglucosyloxybutenenitrile and (Z)-coumaroyloxymethyleneglucosyloxybutenenitrile (section 3.3). The concentrations of these compounds differed significantly between fermentations; the sequentially fermented beverages had significantly higher contents of both compounds than single yeast-fermented beverages. This result is consistent with the reported high β-glucosidase activity of Metschnikowia yeasts.48 In addition, the concentrations of the initial compounds (11 and 12) decreased 1.5–15-fold. The highest decrease was observed with Sc1 by 93.5 and 65%, respectively.
The main flavonols in the black currant juice and beverages were glycosides of myricetin and quercetin. Interestingly, sequential fermentations did have different degradation effects on the flavonols: M1Sc1 and M2Sc1 significantly increased the flavonol aglycon contents, but M1Sc2 did not increase them at all. The total flavonol glycoside contents varied between 79 mg/L (Sc1) and 107 mg/L (M2Sc1; Table 3) in the fermented beverages. The highest content was found in M1Sc1 and M2Sc1, which differed significantly with the one Sc1 beverage, but there were no significant differences between the M1Sc2 and Sc2 beverages. The yeast fermentations did not have a significant effect on the contents of flavan-3-ols. A moderate increase in the catechin content was observed after the sequential fermentations (Table 3).
The color properties, intensity, and tonality of the fermented beverages were analyzed with a spectrophotometer (Table 1). The highest color intensity was recorded from black currant juice. Of the beverages, the Sc2-fermented beverage had the highest color intensity and the Sb beverage had the lowest color intensity. The colors of the beverages produced by sequential fermentations were recorded as more intense than the Sc1, Sb, and Td beverages. A decreased blue color proportion and increased proportion of yellow and red colors were detected in beverages with lower color intensity and lower total anthocyanin contents. After the fermentations, the total anthocyanin contents varied between 637 mg/L (Sb) and 1153 mg/L (M1Sc1; Table 3). Clear differences in the contents of individual monomeric anthocyanins and the total anthocyanins were observed between the sequential and non-sequential fermentations. The total anthocyanin contents of the sequential fermentations did not differ statistically from the non-fermented black currant juice or from each other, whereas all of the fermentations with single yeast strains did. Sb had the lowest total anthocyanin content. The difference between fermentations was in the extent of the decrease in all of the monomeric anthocyanins. Similar to our findings, Belda et al. and Escribano-Viana et al.10,16 have reported higher anthocyanin contents after sequential fermentation with M. pulcherrima and S. cerevisiae compared to S. cerevisiae fermentation. The observed differences in color properties and the loss of monomeric anthocyanin during the fermentations can be attributed to variations in either β-glucosidase activity or anthocyanin absorption by the yeast cell wall among different yeast strains. β-Glucosidase releases the aglycon forms of anthocyanins through the cleavage of the glycosidic bond. Aglycons are not stable and spontaneously convert to colorless and brown compounds. Both β-glucosidase activity and cell wall absorption are yeast species and strain-specific characteristics.14,49−51 Belda et al.16 reported β-glucosidase activity in 88.5% of the studied M. pulcherrima strains and 88.9% of M. fructicola strains.
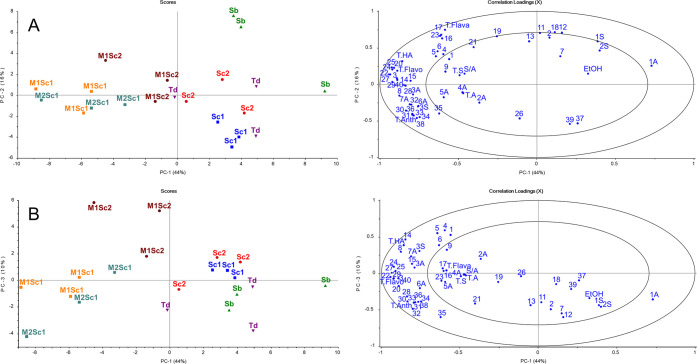
To visualize the relationship between single yeast fermentations and sequential fermentations and between Saccharomyces and non-Saccharomyces beverages, a second PCA was constructed using all 21 fermented beverage samples and 57 chemical variables. The first three PCs are shown in panels A and B of Figure 3 and explain 70% of the data variance. PC1 clearly separates the single yeast fermentations from the sequential fermentations. Sequential fermentations are located on the left side of the scores plot, and the single yeast-fermented beverages are on the right. Between sequential fermentations, there are still clear differences, with M1Sc1 and M2Sc1 located closer to each other than to M1Sc2. M1Sc2 is also separated from M1Sc1 and M2Sc1 on PC2. The correlation loadings plot shows almost all of the chemical variables, especially flavonols and anthocyanins, located on the left side with the Sc1 sequential fermentations. Galacturonic acid (7A) correlates positively with M1Sc2 beverages and clustering most of the anthocyanins and total anthocyanins. This classification can indicate higher pectinase enzyme activity and, thus, positive effects on the contents of phenolic compounds after fermentation. Sc1, Sc2, Td, and Sb beverages are primarily on the right side of the plot. However, the Sb beverages are separated from other single yeast-fermented beverages on PC2. In addition, the majority of chemical variables are correlated positively with Sb, such as Z-p-coumaroyl O-glucoside (6), p-coumaric acid O-glucoside (3), succinic acid (1A), fructose (1S), and glucose (2S). The scores plot also shows that the Sc1, Sc2, and Td beverages are not separated from each other by the differences in the studied chemical variables, and thus, their sensory properties may be more similar to each other than to Sb and sequentially fermented beverages. The only chemical variables positively correlating with Sc1, Sc2, and Td are the delphinidin and cyanidin aglycones and ethanol. The third component separates the M1Sc2 samples from other sequential fermentations and brings the Sb beverages closer to other single yeast-fermented beverages. Additionally, the Sc1 and Sc2 beverages are separated from Td and Sb, indicating that they are more similar to each other than to the other two single yeast fermentations. However, the chemical variables are positively correlated more with Sb and Td. Most interestingly, M1Sc2 clearly correlates negatively with the anthocyanins, initial nitrile compounds (10–13), glucose, fructose, and succinic acid on PC3. The difference between biological replicates of fermentations may be caused by the high pectin content of the black currant juice.
Figure 3.
PCA based on 57 chemical variables of fermented black currant beverages using the following inoculation schemes: (blue ■) S. cerevisiae 1, (red ●) S. cerevisiae 2, (green ▲) S. bayanus, (purple ▼) T. delbrueckii, (orange ■) M. pulcherrima + S. cerevisiae 1, (cyan ■) M. fructicola + S. cerevisiae 1, and (maroon ●) M. pulcherrima + S. cerevisiae 2 for (A) PC1 versus PC2 and (B) PC1 versus PC3. Numbers with A (acid) or S (sugar) refer to Table 1, and the numbers of variables refer to Table 3. T.HA, total hydroxycinnamic acids; T.Flava, total flavan-3-ols; T.Flavo, total flavonols; T.Anth, total anthocyanins; T.A, total organic acids; T.S, total sugars; and S/A, sugar/acid ratio.
In conclusion, although black currant berries and juices are widely studied,3,6,7,20 studies on the composition of black currant fermented beverages are scarce and focused only on fermentations with S. cerevisiae.21,22,24,25,27 To our knowledge, this is the first study on the effects of non-Saccharomyces yeast fermentations on the chemical composition of the fermented black currant beverages. All of the fermentations decreased the content of phenolic acids containing nitrile moieties. Sequential fermentations increased the contents of hydroxycinnamic acids, flavan-3-ols, and flavonols but decreased the ethanol content compared to single yeast fermentations. Beverages fermented with S. cerevisiae or T. delbrueckii alone were similar to each other, whereas S. bayanus was significantly different from the other single yeast-fermented beverages. The S. bayanus beverage had the lowest amounts of anthocyanins, and its color intensity was significantly lower than that of all of the other fermented beverages. When three sequential fermentations were compared to each other, it was clear that the S. cerevisiae strain also effects the concentrations of phenolic compounds; fermentations with either Metschnikowia yeast or S. cerevisiae strain 1 resulted primarily in more of phenolic compounds than fermentation with M. pulcherrima and S. cerevisiae strain 2. Sequential fermentation with Metschnikowia and S. cerevisiae yeasts could be considered to be the most suitable for black currant juice, if the aim is to maximize the preservation of the phenolic compounds and to gain the highest sugar/acid ratio. In this sense, single yeast fermentations would be more suitable for higher ethanol production. In addition, S. bayanus was the least suitable yeast to ferment black currant juice as a result of its high succinic acid production, low phenolic compound concentrations, and clear decrease in color properties.
This study demonstrated the potential use of Saccharomyces and non-Saccharomyces yeasts in modifying the chemical composition of black currant beverages. However, there is still a gap in knowledge about how fermentations with different yeasts affect the sensory properties of fermented black currant beverages. Moreover, the fermented black currant beverages could be intensely sour as a result of the sugar utilization by the yeasts and increased acid content. Further studies are necessary to study how wine yeast fermentations affect the aroma profile of black currant juice.
This work was supported by the Ella and George Ehnrooth Foundation and the Finnish Cultural Foundation (00190487, 2019).
The authors declare no competing financial interest.
References
- Tian Y.; Laaksonen O.; Haikonen H.; Vanag A.; Ejaz H.; Linderborg K.; Karhu S.; Yang B. Compositional Diversity among Blackcurrant (Ribes nigrum) Cultivars Originating from European Countries. J. Agric. Food Chem. 2019, 67 (19), 5621–5633. 10.1021/acs.jafc.9b00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez R. E.; Gonzalez de Mejia E. Blackcurrants (Ribes nigrum): A Review on Chemistry, Processing, and Health Benefits. J. Food Sci. 2019, 84 (9), 2387–2401. 10.1111/1750-3841.14781. [DOI] [PubMed] [Google Scholar]
- Laaksonen O. A.; Mäkilä L.; Sandell M. A.; Salminen J.-P.; Liu P.; Kallio H. P.; Yang B. Chemical-Sensory Characteristics and Consumer Responses of Blackcurrant Juices Produced by Different Industrial Processes. Food Bioprocess Technol. 2014, 7 (10), 2877–2888. 10.1007/s11947-014-1316-8. [DOI] [Google Scholar]
- de Pascual-Teresa S.; Moreno D. A.; García-Viguera C. Flavanols and Anthocyanins in Cardiovascular Health: A Review of Current Evidence. Int. J. Mol. Sci. 2010, 11 (4), 1679–1703. 10.3390/ijms11041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szajdek A.; Borowska E. J. Bioactive Compounds and Health-Promoting Properties of Berry Fruits: A Review. Plant Foods Hum. Nutr. 2008, 63 (4), 147–156. 10.1007/s11130-008-0097-5. [DOI] [PubMed] [Google Scholar]
- Nour V.; Stampar F.; Veberic R.; Jakopic J. Anthocyanins Profile, Total Phenolics and Antioxidant Activity of Black Currant Ethanolic Extracts as Influenced by Genotype and Ethanol Concentration. Food Chem. 2013, 141 (2), 961–966. 10.1016/j.foodchem.2013.03.105. [DOI] [PubMed] [Google Scholar]
- Tian Y.; Liimatainen J.; Alanne A.-L.; Lindstedt A.; Liu P.; Sinkkonen J.; Kallio H.; Yang B. Phenolic Compounds Extracted by Acidic Aqueous Ethanol from Berries and Leaves of Different Berry Plants. Food Chem. 2017, 220, 266–281. 10.1016/j.foodchem.2016.09.145. [DOI] [PubMed] [Google Scholar]
- Sun S. Y.; Gong H. S.; Zhao Y. P.; Liu W. L.; Jin C. W. Sequential Culture with Torulaspora delbrueckii and Saccharomyces cerevisiae and Management of Fermentation Temperature to Improve Cherry Wine Quality. J. Sci. Food Agric. 2016, 96 (6), 1880–1887. 10.1002/jsfa.7293. [DOI] [PubMed] [Google Scholar]
- Contreras A.; Hidalgo C.; Henschke P. A.; Chambers P. J.; Curtin C.; Varela C. Evaluation of Non-Saccharomyces Yeasts for the Reduction of Alcohol Content in Wine. Appl. Environ. Microbiol. 2014, 80 (5), 1670–1678. 10.1128/AEM.03780-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano-Viana R.; Portu J.; Garijo P.; López R.; Santamaría P.; López-Alfaro I.; Gutiérrez A. R.; González-Arenzana L. Effect of the Sequential Inoculation of Non-Saccharomyces/Saccharomyces on the Anthocyans and Stilbenes Composition of Tempranillo Wines. Front. Microbiol. 2019, 10, 10. 10.3389/fmicb.2019.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loira I.; Morata A.; Comuzzo P.; Callejo M. J.; González C.; Calderón F.; Suárez-Lepe J. A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii Strains in Mixed and Sequential Fermentations to Improve Red Wine Sensory Quality. Food Res. Int. 2015, 76, 325–333. 10.1016/j.foodres.2015.06.030. [DOI] [PubMed] [Google Scholar]
- Morata A.; Escott C.; Loira I.; Del Fresno J. M.; González C.; Suárez-Lepe J. A. Influence of Saccharomyces and Non-Saccharomyces Yeasts in the Formation of Pyranoanthocyanins and Polymeric Pigments during Red Wine Making. Molecules 2019, 24 (24), 4490–4508. 10.3390/molecules24244490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asenstorfer R. E.; Markides A. J.; Iland P. G.; Jones G. P. Formation of Vitisin A during Red Wine Fermentation and Maturation. Aust. J. Grape Wine Res. 2003, 9 (1), 40–46. 10.1111/j.1755-0238.2003.tb00230.x. [DOI] [Google Scholar]
- Hong M.; Li J.; Chen Y. Characterization of Tolerance and Multi-Enzyme Activities in Non-Saccharomyces Yeasts Isolated from Vidal Blanc Icewine Fermentation. J. Food Biochem. 2019, 43 (11), e13027. 10.1111/jfbc.13027. [DOI] [PubMed] [Google Scholar]
- Manzanares P.; Rojas V.; Genovés S.; Vallés S. A Preliminary Search for Anthocyanin-β-d-Glucosidase Activity in Non-Saccharomyces Wine Yeasts. Int. J. Food Sci. Technol. 2000, 35 (1), 95–103. 10.1046/j.1365-2621.2000.00364.x. [DOI] [Google Scholar]
- Belda I.; Conchillo L. B.; Ruiz J.; Navascués E.; Marquina D.; Santos A. Selection and Use of Pectinolytic Yeasts for Improving Clarification and Phenolic Extraction in Winemaking. Int. J. Food Microbiol. 2016, 223, 1–8. 10.1016/j.ijfoodmicro.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Pogorzelski E.; Wilkowska A. Flavour Enhancement through the Enzymatic Hydrolysis of Glycosidic Aroma Precursors in Juices and Wine Beverages: A Review. Flavour Fragrance J. 2007, 22 (4), 251–254. 10.1002/ffj.1784. [DOI] [Google Scholar]
- Bender C.; Killermann K. V.; Rehmann D.; Weidlich H. H. Effect of Mash Enzyme and Heat Treatments on the Cellular Antioxidant Activity of Black Currant (Ribes nigrum), Raspberry (Rubus idaeus), and Blueberry (Vaccinium myrtillus) Juices. CyTA—J. Food 2017, 15 (2), 277–283. 10.1080/19476337.2016.1247914. [DOI] [Google Scholar]
- Koponen J. M.; Happonen A. M.; Auriola S.; Kontkanen H.; Buchert J.; Poutanen K. S.; Törrönen A. R. Characterization and Fate of Black Currant and Bilberry Flavonols in Enzyme-Aided Processing. J. Agric. Food Chem. 2008, 56 (9), 3136–3144. 10.1021/jf703676m. [DOI] [PubMed] [Google Scholar]
- Mäkilä L.; Laaksonen O.; Alanne A.-L.; Kortesniemi M.; Kallio H.; Yang B. Stability of Hydroxycinnamic Acid Derivatives, Flavonol Glycosides, and Anthocyanins in Black Currant Juice. J. Agric. Food Chem. 2016, 64 (22), 4584–4598. 10.1021/acs.jafc.6b01005. [DOI] [PubMed] [Google Scholar]
- Czyżowska A.; Pogorzelski E. Changes to Polyphenols in the Process of Production of Must and Wines from Blackcurrants and Cherries. Part II. Anthocyanins and Flavanols. Eur. Food Res. Technol. 2004, 218 (4), 355–359. 10.1007/s00217-003-0857-2. [DOI] [Google Scholar]
- Czyzowska A.; Pogorzelski E. Changes to Polyphenols in the Process of Production of Must and Wines from Blackcurrants and Cherries. Part I. Total Polyphenols and Phenolic Acids. Eur. Food Res. Technol. 2002, 214 (2), 148–154. 10.1007/s00217-001-0422-9. [DOI] [Google Scholar]
- Heinonen I. M.; Lehtonen P. J.; Hopia A. I. Antioxidant Activity of Berry and Fruit Wines and Liquors. J. Agric. Food Chem. 1998, 46 (1), 25–31. 10.1021/jf970489o. [DOI] [PubMed] [Google Scholar]
- Leino M.; Kallio H. Volatile Compounds of Blackcurrant Juice and Wine. Z. Lebensm.-Unters. Forsch. 1993, 196 (5), 410–414. 10.1007/BF01190803. [DOI] [Google Scholar]
- Rupasinghe H. P. V.; Clegg S. Total Antioxidant Capacity, Total Phenolic Content, Mineral Elements, and Histamine Concentrations in Wines of Different Fruit Sources. J. Food Compos. Anal. 2007, 20 (2), 133–137. 10.1016/j.jfca.2006.06.008. [DOI] [Google Scholar]
- Vuorinen H.; Määttä K.; Törrönen R. Content of the Flavonols Myricetin, Quercetin, and Kaempferol in Finnish Berry Wines. J. Agric. Food Chem. 2000, 48 (7), 2675–2680. 10.1021/jf991388o. [DOI] [PubMed] [Google Scholar]
- Wilkowska A.; Czyżowska A.; Ambroziak W.; Adamiec J. Structural, Physicochemical and Biological Properties of Spray-Dried Wine Powders. Food Chem. 2017, 228, 77–84. 10.1016/j.foodchem.2017.01.115. [DOI] [PubMed] [Google Scholar]
- Laaksonen O.; Sandell M.; Nordlund E.; Heiniö R.-L.; Malinen H.-L.; Jaakkola M.; Kallio H. The Effect of Enzymatic Treatment on Blackcurrant (Ribes nigrum) Juice Flavour and Its Stability. Food Chem. 2012, 130 (1), 31–41. 10.1016/j.foodchem.2011.06.048. [DOI] [Google Scholar]
- Kelanne N.; Laaksonen O.; Seppälä T.; Yang W.; Tuukkanen K.; Loponen J.; Yang B. Impact of Cyclodextrin Treatment on Composition and Sensory Properties of Lingonberry (Vaccinium vitis-idaea) Juice. LWT 2019, 113, 108295. 10.1016/j.lwt.2019.108295. [DOI] [Google Scholar]
- Liu S.; Laaksonen O.; Kortesniemi M.; Kalpio M.; Yang B. Chemical Composition of Bilberry Wine Fermented with Non-Saccharomyces Yeasts (Torulaspora delbrueckii and Schizosaccharomyces pombe) and Saccharomyces cerevisiae in Pure, Sequential and Mixed Fermentations. Food Chem. 2018, 266, 262–274. 10.1016/j.foodchem.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Satora P.; Semik-Szczurak D.; Tarko T.; Bułdys A. Influence of Selected Saccharomyces and Schizosaccharomyces Strains and Their Mixed Cultures on Chemical Composition of Apple Wines. J. Food Sci. 2018, 83 (2), 424–431. 10.1111/1750-3841.14042. [DOI] [PubMed] [Google Scholar]
- Gawkowska D.; Cieśla J.; Zdunek A.; Cybulska J. The Effect of Concentration on the Cross-Linking and Gelling of Sodium Carbonate-Soluble Apple Pectins. Molecules 2019, 24 (8), 1635. 10.3390/molecules24081635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano-Viana R.; González-Arenzana L.; Portu J.; Garijo P.; López-Alfaro I.; López R.; Santamaría P.; Gutiérrez A. R. Wine Aroma Evolution throughout Alcoholic Fermentation Sequentially Inoculated with Non-Saccharomyces/Saccharomyces Yeasts. Food Res. Int. 2018, 112, 17–24. 10.1016/j.foodres.2018.06.018. [DOI] [PubMed] [Google Scholar]
- Contreras A.; Hidalgo C.; Schmidt S.; Henschke P. A.; Curtin C.; Varela C. The Application of Non-Saccharomyces Yeast in Fermentations with Limited Aeration as a Strategy for the Production of Wine with Reduced Alcohol Content. Int. J. Food Microbiol. 2015, 205, 7–15. 10.1016/j.ijfoodmicro.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Legan J. D.; Voysey P. A. Yeast Spoilage of Bakery Products and Ingredients. J. Appl. Bacteriol. 1991, 70 (5), 361–371. 10.1111/j.1365-2672.1991.tb02950.x. [DOI] [PubMed] [Google Scholar]
- Aneta W.; Jan O.; Magdalena M.; Joanna W. Phenolic Profile, Antioxidant and Antiproliferative Activity of Black and Red Currants (Ribes spp.) from Organic and Conventional Cultivation. Int. J. Food Sci. Technol. 2013, 48 (4), 715–726. 10.1111/ijfs.12019. [DOI] [Google Scholar]
- da Silva F. L.; Escribano-Bailón M. T.; Pérez Alonso J. J.; Rivas-Gonzalo J. C.; Santos-Buelga C. Anthocyanin Pigments in Strawberry. LWT - Food Sci. Technol. 2007, 40 (2), 374–382. 10.1016/j.lwt.2005.09.018. [DOI] [Google Scholar]
- de Villiers A.; Vanhoenacker G.; Majek P.; Sandra P. Determination of Anthocyanins in Wine by Direct Injection Liquid Chromatography–Diode Array Detection–Mass Spectrometry and Classification of Wines Using Discriminant Analysis. J. Chromatogr. A 2004, 1054 (1-2), 195–204. 10.1016/j.chroma.2004.07.087. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Yeap Foo L.; Wong H. Nigrumin-5-p-Coumarate and Nigrumin-5-Ferulate, Two Unusual Nitrile-Containing Metabolites from Black Currant (Ribes nigrum) Seed. Phytochemistry 2002, 59 (4), 465–468. 10.1016/S0031-9422(01)00441-1. [DOI] [PubMed] [Google Scholar]
- Ruta L. L.; Farcasanu I. C. Anthocyanins and Anthocyanin-Derived Products in Yeast-Fermented Beverages. Antioxidants 2019, 8 (6), 182. 10.3390/antiox8060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribéreau-Gayon P.; Dubourdieu D.; Donèche B.; Lonvaud A.. Handbook of Enology, The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, 2005; Vol. 1, 10.1002/0470010363. [DOI] [Google Scholar]
- Mikulic-Petkovsek M.; Koron D.; Veberic R. Quality Parameters of Currant Berries from Three Different Cluster Positions. Sci. Hortic. 2016, 210, 188–196. 10.1016/j.scienta.2016.07.030. [DOI] [Google Scholar]
- Raab A. M.; Lang C. Oxidative versus Reductive Succinic Acid Production in the Yeast Saccharomyces cerevisiae. Bioeng. Bugs 2011, 2 (2), 120–123. 10.4161/bbug.2.2.14549. [DOI] [PubMed] [Google Scholar]
- Eglinton J. M.; McWILLIAM S. J.; Fogarty M. W.; Francis I. L.; Kwiatkowski M. J.; Høj P. B.; Henschke P. A. The Effect of Saccharomyces bayanus-Mediated Fermentation on the Chemical Composition and Aroma Profile of Chardonnay Wine. Aust. J. Grape Wine Res. 2000, 6 (3), 190–196. 10.1111/j.1755-0238.2000.tb00178.x. [DOI] [Google Scholar]
- Canonico L.; Comitini F.; Oro L.; Ciani M. Sequential Fermentation with Selected Immobilized Non-Saccharomyces Yeast for Reduction of Ethanol Content in Wine. Front. Microbiol. 2016, 7, 11. 10.3389/fmicb.2016.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Arneborg N.; Toldam-Andersen T. B.; Petersen M. A.; Bredie W. L. Effect of Sequential Fermentations and Grape Cultivars on Volatile Compounds and Sensory Profiles of Danish Wines. J. Sci. Food Agric. 2017, 97 (11), 3594–3602. 10.1002/jsfa.8218. [DOI] [PubMed] [Google Scholar]
- Rubico S. M.; McDaniel M. R. Sensory Evaluation of Acids by Free-Choice Profiling. Chem. Senses 1992, 17 (3), 273–289. 10.1093/chemse/17.3.273. [DOI] [Google Scholar]
- Carlson M. Regulation of Sugar Utilization in Saccharomyces Species. J. Bacteriol. 1987, 169 (11), 4873–4877. 10.1128/JB.169.11.4873-4877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morata A.; Loira I.; Escott C.; del Fresno J. M.; Bañuelos M. A.; Suárez-Lepe J. A. Applications of Metschnikowia pulcherrima in Wine Biotechnology. Fermentation 2019, 5 (3), 63. 10.3390/fermentation5030063. [DOI] [Google Scholar]
- Blazquez Rojas I.; Smith P. A.; Bartowsky E. J. Influence of Choice of Yeasts on Volatile Fermentation-Derived Compounds, Colour and Phenolics Composition in Cabernet Sauvignon Wine. World J. Microbiol. Biotechnol. 2012, 28 (12), 3311–3321. 10.1007/s11274-012-1142-y. [DOI] [PubMed] [Google Scholar]
- Morata A.; Gómez-Cordovés M. C.; Colomo B.; Suárez J. A. Cell Wall Anthocyanin Adsorption by Different Saccharomyces Strains during the Fermentation of Vitis vinifera L. Cv Graciano Grapes. Eur. Food Res. Technol. 2005, 220 (3), 341–346. 10.1007/s00217-004-1053-8. [DOI] [Google Scholar]