Abstract
Background
Patients hospitalized with heart failure (HF) with reduced ejection fraction have high risk of rehospitalization or death. Despite guideline recommendations based on high‐quality evidence, a substantial proportion of patients with HF with reduced ejection fraction receive suboptimal care and/or do not comply with optimal care following hospitalization.
Methods and Results
This retrospective observational study identified 17 106 patients with HF with reduced ejection fraction with an incident HF‐related hospitalization using the Humana Medicare Advantage database (2008–2016). HF medication classes (beta‐blockers, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, angiotensin receptor neprilysin inhibitors, or mineralocorticoid receptor antagonists) received in the year after hospitalization were recorded, and categorized by treatment intensity (ie, number of concomitant medication classes received: none [23% of patients; n=3987], monotherapy [22%; n=3777], dual therapy [41%; n=7056], or triple therapy [13%; n=2286]). Compared with no medication, risk of primary outcome (composite of death or rehospitalization) was significantly reduced (hazard ratio [95% CI]) with monotherapy (0.68 [0.64–0.71]), dual therapy (0.56 [0.53–0.59]), and triple therapy (0.45 [0.41–0.50]). Nearly half (46%) of patients who received post‐discharge medication had no dose escalation. Overall, 59% of patients had follow‐up with a primary care physician within 14 days of discharge, and 23% had follow‐up with a cardiologist.
Conclusions
In real‐world clinical practice, increasing treatment intensity reduced risk of death and rehospitalization among patients hospitalized for HF, though the use of guideline‐recommended dual and triple HF therapy remained low. There are opportunities to improve post‐discharge medical management for patients with HF with reduced ejection fraction such as optimizing dose titration and improving post‐discharge follow‐up with providers.
Keywords: heart failure (with reduced ejection fraction), mortality, outcomes, rehospitalization, treatment intensity
Subject Categories: Heart Failure, Treatment
Nonstandard Abbreviations and Acronyms
- ACEI
angiotensin‐converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- ARNI
angiotensin receptor neprilysin inhibitor
- BB
beta‐blocker
- ED
emergency department
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- ICD‐10‐CM
International Classification of Diseases, Tenth Revision, Clinical Modification
- ICD‐9‐CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- MRA
mineralocorticoid receptor antagonist
- PCP
primary care physician
- PPY
per person‐year
Clinical Perspective
What Is New?
In patients hospitalized with heart failure (HF) with reduced ejection fraction in the United States, outpatient use of guideline‐directed HF therapy in the year after hospital discharge remains low (around 54%).
Guideline‐directed use of dual or triple HF therapy in outpatients with HF with reduced ejection fraction is associated with reduced 1‐year mortality and rehospitalization.
What Are the Clinical Implications?
Owing to inadequate use of recommended outpatient HF therapies in the United States, clinical outcomes appear to be suboptimal among patients with HF with reduced ejection fraction.
Heart failure (HF) is a disabling, progressive clinical syndrome characterized by inadequate ventricular filling or ejection of blood. 1 In the United States, >650 000 people are diagnosed with HF annually, and incidence increases with age 2 ; estimated 5‐year mortality after diagnosis is ≈50%. 3 , 4 Over 20% of patients have an HF‐related hospitalization in the year after diagnosis. 5 , 6 Outcomes following HF‐related hospitalization are poor; within 30 days of hospital discharge, 25% of patients are readmitted, and 10% of patients die. 7 , 8
Approximately half of patients with HF have a reduced ejection fraction (HFrEF), characterized by systolic dysfunction and defined clinically by a left‐ventricular ejection fraction ≤40%. 1 The American College of Cardiology Foundation/American Heart Association guidelines recommend that patients with HFrEF receive dual therapy with beta‐blockers (BBs) in combination with angiotensin‐converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), or angiotensin receptor neprilysin inhibitors (ARNIs) if patients tolerate ACEIs or ARBs. 1 , 9 , 10 In patients who remain symptomatic despite these therapies, the addition of mineralocorticoid receptor antagonists (MRAs) is recommended as triple therapy. Diuretics are used for symptomatic management of fluid retention. Most patients with HFrEF (>98%) are candidates for guideline‐directed medical therapy, having no absolute contraindications to treatment. 11 Despite the availability and efficacy of these therapies, suboptimal use in real‐world clinical practice remains high. 11 , 12 , 13 As intolerance accounts for only ≈5% to 20% of underutilization in treated patients, 14 the need for prudent initiation and uptitration of HF therapies persists.
American College of Cardiology Foundation/American Heart Association guidelines also provide clear direction for optimal care of patients with HF during and after hospitalization. If the patient is not already receiving HF medication before hospitalization, guidelines highlight the need for prompt initiation and subsequent optimization of chronic HF therapy. 1 After discharge from the hospital, routine, cautious titration of medication in tandem with continued patient observation is essential to adjust therapy appropriately. 9 Despite this guidance, after discharge >60% of patients with HF had no change to their pre‐admission therapy. 5 Early outpatient follow‐up within 7 to 14 days of discharge is also strongly recommended, 1 and has been associated with a lower risk of subsequent rehospitalization. 15 This holistic approach, incorporating optimal medication strategies in tandem with regular clinical follow‐up, is an important component of transitional care, and provides an opportunity to evaluate and adjust the care plan of the patient after discharge from hospital.
Better understanding of post‐discharge treatment patterns and early outpatient follow‐up may inform opportunities for intervention to improve clinical management for patients with HFrEF. The purpose of this study was to assess real‐world clinical outcomes, treatment patterns, and post‐discharge care in patients with HFrEF who experienced an incident HF‐related hospitalization.
METHODS
The authors declare that supporting research materials and analytical methods relevant to the conduct of this study are available within the article (and its online supplementary files). The source data files will not be made available to other researchers for purposes of replicating analysis procedures or reproducing study results.
Study Population
This retrospective cohort study used the Humana Research Database (Louisville, KY), a repository of administrative claims, enrollment, and provider data relating to patients enrolled in the Humana Medicare Advantage Prescription Drug plan. Patients aged 65 to 89 years with newly diagnosed HFrEF and a record of incident HF‐related hospitalization between January 1, 2008 and March 31, 2016 were included in the study. Cases of HFrEF were defined using a previously‐validated claims‐based algorithm, shown to have excellent specificity and predictive value. 16 Patients with HFrEF were identified by the presence of 2 outpatient medical claims (within 30–365 days of each other) or 1 inpatient medical claim with codes that specified systolic HF, identified by International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification (ICD‐9‐CM or ICD‐10‐CM) codes 428.2 or I50.2 (systolic HF) or 428.4 or I50.4 (combined systolic and diastolic HF). The index date was the discharge date of the first hospitalization with HF as the principal diagnosis (ICD‐9‐CM code 428 or ICD‐10‐CM code I50). Patients who died during this hospitalization were excluded. Continuous enrollment with medical and pharmacy benefits, without a 90‐day stay in a long‐term care facility, was required for at least 12 months pre‐index. Newly diagnosed HFrEF was identified by excluding patients with medical claims relating to HF in the 12 months on or before the pre‐index diagnosis. Data from all eligible patients were collected from 1 year before the index date until the earliest of disenrollment, death, or study end date (March 31, 2017, to ensure a minimum of 12 months' potential follow‐up). Data were obtained from the Humana Research Database, including information on demographics, enrollment, encounters, inpatient and outpatient diagnoses and procedures, pharmacy fills, and death. The Schulman Institutional Review Board approved this study; as this was a retrospective observational study using a limited administrative data set, informed consent was waived by the Institutional Review Board.
Exposure
Pharmacy claims data were used to identify medication classes of HF therapy (ACEIs/ARBs/ARNIs, BBs, and MRAs) dispensed to patients in the year after hospital discharge. To be categorized as having received these medication classes, patients were required to have at least 2 fills within any 6‐month interval post‐index, or 1 fill for a 90‐day supply for any medication within that class in the year after hospital discharge. Continuous use was defined as no gaps of ≥60 days between the predicted end date of a prescription supply and the fill date of the subsequent fill for medications within the same medication class. To qualify as concurrent use, the days' supply for fills from different classes must have overlapped for at least 28 consecutive days. Treatment intensity was defined by the maximal number of concurrent medication classes dispensed during the year after hospital discharge. We categorized treatment intensity as: no HF therapy; monotherapy (ACEI/ARB/ARNI or BB or MRA); dual therapy ([ACEI/ARB/ARNI+BB] or [MRA+BB] or [ACEI/ARB/ARNI+MRA]); or triple therapy (BB+ACEI/ARB/ARNI+MRA). 5
Outcome Measures
The primary outcome was time from discharge until the earliest occurrence of either death or rehospitalization of any cause. All‐cause death was identified using date of death on the enrollment file. All‐cause rehospitalizations were defined as a record of inpatient stay within the year after index hospital discharge. Visits to the emergency department (ED) were identified using revenue codes, place of treatment codes, and Common Procedural Terminology/Healthcare Common Procedure Coding System codes; those ED visits resulting in inpatient admission were counted as hospitalizations.
Post‐discharge follow‐up care was evaluated by patient encounters with a primary care physician (PCP) or cardiologist within 7, 14, and 30 days of hospital discharge. Post‐discharge medication doses were obtained from pharmacy fill records. Initial dose was the dose of the first fill after discharge; maintenance dose was identified as the first 2 consecutive identical prescription fills occurring >90 days post‐discharge. Maintenance dose was categorized based on target doses recommended by clinical practice guidelines, 1 , 9 and stratified as <50% target dose, 50% to <75% target dose, and ≥75% target dose. Titration was assessed as the number of dose adjustments from the initial dose. Prescriber specialty was identified from the first pharmacy claim for each medication class, and categorized as PCP, cardiologist, nurse practitioner/physician assistant, or other specialty.
Statistical Analysis
Baseline patient characteristics were reported descriptively using standard summary statistics. Categorical variables were reported as frequency counts and percentages and compared using Chi‐square tests; continuous variables were reported as means and SDs and were compared using t tests.
We used multivariable Cox proportional hazards models to derive hazard ratios (HRs), 95% CIs, and cumulative incidence function curves assessing the association of treatment intensity and the primary (death or rehospitalization) composite outcome in the year after hospital discharge, while accounting for competing risks. Patients were followed‐up for 1 year after discharge, or until the earliest of composite outcome or disenrollment. Treatment intensity was modeled as a time‐varying exposure. The treatment intensity category most proximal to the event/censor was used. Patients receiving no therapy served as the reference group in all analyses. All models were adjusted for age, sex, race, geographic region, Deyo‐Charlson comorbidity index, presence of hypertension, dyslipidemia, prior stroke, atherosclerosis, atrial fibrillation, peripheral vascular disease, diabetes mellitus, renal disease, chronic obstructive pulmonary disease, and prior use of HF medications. Analyses were conducted using SAS Enterprise Guide version 7.11 (SAS Institute Inc, Cary, NC).
RESULTS
Baseline Demographics and Disease Characteristics
In total, 17 106 patients with incident hospitalization for newly diagnosed HFrEF were included. The mean age of the overall cohort at index was 77 years, and most patients were men (60%), White (83%), from the south of the United States (64%), and had high baseline comorbidity (mean Deyo‐Charlson comorbidity index [SD]: 4.7 [2.5]) (Table 1). Patients who did not receive any of the 3 HF medication classes post‐discharge (ACEI/ARB/ARNI, BB, or MRA) were more likely to be White (87% versus 81%), men (67% versus 58%), and aged >85 years (25% versus 16%) than those who did receive medication. Nearly a quarter of all patients received no post‐discharge medications, and nearly half did not receive dual or triple therapy. Mean follow‐up time ranged from a mean (SD) of 173 (153) days (no post‐discharge medication) to 339 (68) days (triple therapy).
Table 1.
Baseline Characteristics for Patients With HFrEF by Treatment Intensity
|
No Medication n=3987 |
Monotherapy n=3777 |
Dual Therapy n=7056 |
Triple Therapy n=2286 |
|
|---|---|---|---|---|
| Mean follow‐up, d (SD) | 173 (153) | 293 (112) | 326 (84) | 339 (68) |
| Mean age, y (SD) | 78.9 (6.6) | 78.0 (6.6) | 76.8 (6.5) | 75.6 (6.3) |
| 65–69 y, n (%) | 428 (10.7) | 444 (11.8) | 1174 (16.6) | 458 (20.0) |
| 70–74 y, n (%) | 694 (17.4) | 822 (21.8) | 1697 (24.1) | 621 (27.2) |
| 75–79 y, n (%) | 870 (21.8) | 830 (22.0) | 1581 (22.4) | 541 (23.7) |
| 80–84 y, n (%) | 1009 (25.3) | 905 (24.0) | 1521 (21.6) | 418 (18.3) |
| 85–89 y, n (%) | 986 (24.7) | 776 (20.5) | 1083 (15.3) | 248 (10.8) |
| Sex, n (%) | ||||
| Men | 2684 (67.3) | 2267 (60.0) | 3992 (56.6) | 1306 (57.1) |
| Race, n (%) | ||||
| White | 3475 (87.2) | 3116 (82.5) | 5766 (81.7) | 1782 (78.0) |
| Black | 410 (10.3) | 538 (14.2) | 1052 (14.9) | 417 (18.2) |
| Asian, Hispanic, Native American, Pacific Islander | 86 (2.2) | 103 (2.7) | 187 (2.7) | 66 (2.9) |
| Unknown | 16 (0.4) | 20 (0.5) | 51 (0.7) | 21 (0.9) |
| Geographic region, n (%) | ||||
| South | 2487 (62.4) | 2385 (63.1) | 4510 (63.9) | 1421 (62.2) |
| Midwest | 1071 (26.9) | 1000 (26.5) | 1824 (25.9) | 593 (25.9) |
| West | 286 (7.2) | 298 (7.9) | 554 (7.9) | 212 (9.3) |
| Northeast | 143 (3.6) | 94 (2.5) | 168 (2.4) | 60 (2.6) |
| Medical history, n (%) | ||||
| Hypertension | 3690 (92.6) | 3589 (95.0) | 6697 (94.9) | 2149 (94.0) |
| Dyslipidemia | 3060 (76.7) | 3073 (81.4) | 5788 (82.0) | 1850 (80.9) |
| Obesity | 576 (14.4) | 625 (16.5) | 1287 (18.2) | 458 (20.0) |
| Stroke | 941 (23.6) | 954 (25.3) | 1576 (22.3) | 477 (20.9) |
| Atherosclerosis | 926 (23.2) | 915 (24.2) | 1539 (21.8) | 475 (20.8) |
| Myocardial infarction | 850 (21.3) | 713 (18.9) | 1342 (19.0) | 444 (19.4) |
| Atrial fibrillation | 2191 (55.0) | 2033 (53.8) | 3449 (48.9) | 1058 (46.3) |
| Peripheral vascular disease | 1047 (26.3) | 1040 (27.5) | 1668 (23.6) | 515 (22.5) |
| Diabetes mellitus | 2026 (50.8) | 2069 (54.8) | 3874 (54.9) | 1243 (54.4) |
| Renal disease | 2088 (52.4) | 2160 (57.2) | 3037 (43.0) | 765 (33.5) |
| COPD | 2126 (53.3) | 1873 (49.6) | 3249 (46.0) | 1035 (45.3) |
| Mean Deyo‐Charlson index score, SD | 5.0 (2.7) | 4.9 (2.6) | 4.5 (2.5) | 4.2 (2.4) |
| Baseline medications, n (%) | ||||
| ACEI | 1180 (29.6) | 1614 (42.7) | 4074 (57.7) | 1311 (57.3) |
| ARB | 344 (8.6) | 672 (17.8) | 1521 (21.6) | 517 (22.6) |
| BB | 1583 (39.7) | 2429 (64.3) | 5081 (72.0) | 1575 (68.9) |
| MRA | 284 (7.1) | 312 (8.3) | 559 (7.9) | 506 (22.1) |
| Diuretics | 1892 (47.5) | 2630 (69.6) | 4801 (68.0) | 1598 (69.9) |
| Digoxin/digitoxin | 323 (8.1) | 463 (12.3) | 863 (12.2) | 266 (11.6) |
| Hydralazine/isosorbide dinitrate | 483 (12.1) | 759 (20.1) | 1143 (16.2) | 308 (13.5) |
All baseline categories were significant (P<0.001) except the comorbidity of atherosclerosis, which had a P value of 0.0525. Column headings denote HF medication use in the year post‐discharge. No medication: no ACEI/ARB/ARNI, BB, or MRA; monotherapy: ACEI/ARB/ARNI or BB or MRA; dual therapy: (ACEI/ARB/ARNI+BB) or (MRA+BB) or (ACEI/ARB/ARNI+MRA); triple therapy (BB+ACEI/ARB/ARNI+MRA). "Baseline medication” denotes medication use pre‐index. Pre‐index ARNI use was <1% (possibly attributable to the time period of the analysis, 2008–2016). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta‐blocker; COPD, chronic obstructive pulmonary disease; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; and MRA, mineralocorticoid receptor antagonist.
Clinical Outcomes and Healthcare Resource Utilization
Compared with no HF medication, treatment intensification was associated with a progressive improvement in the primary composite outcome (Figure 1A), with a 32% reduction in incidence of death or rehospitalization with monotherapy (n=3777; HR, 0.68 [95% CI, 0.64–0.71]), a 44% reduction with dual therapy (n=7056; HR, 0.56 [95% CI, 0.53–0.59]), and a 55% reduction with triple therapy (n=2286; HR, 0.45 [95% CI, 0.41–0.50]). HRs from the full Cox regression model are presented in Table S1. Incidence curves for the death and rehospitalization components of the primary outcome are also presented (Figure 1B and 1C); both demonstrated improvement with treatment intensification. Notably, the incidence of these outcomes appeared to increase most rapidly during the first 100 days or so of observation, particularly in patients who received no medication. Mortality was higher in patients who received no post‐discharge medication (20.5 deaths per person‐year [PPY]) than in patients prescribed monotherapy (1.1 deaths PPY), dual therapy (0.4 death PPY), or triple therapy (0.2 death PPY) (Table 2). This pattern was also observed when assessing incidence of rehospitalization and ED visits. Length of stay during rehospitalization was longer among patients who received no medication (7.7 days) than among patients prescribed HF medication (monotherapy, 6.6 days; dual therapy, 5.8 days; triple therapy, 5.4 days).
Figure 1. Cumulative incidence of (A) primary composite outcome; (B) all‐cause death; (C) all‐cause rehospitalization.
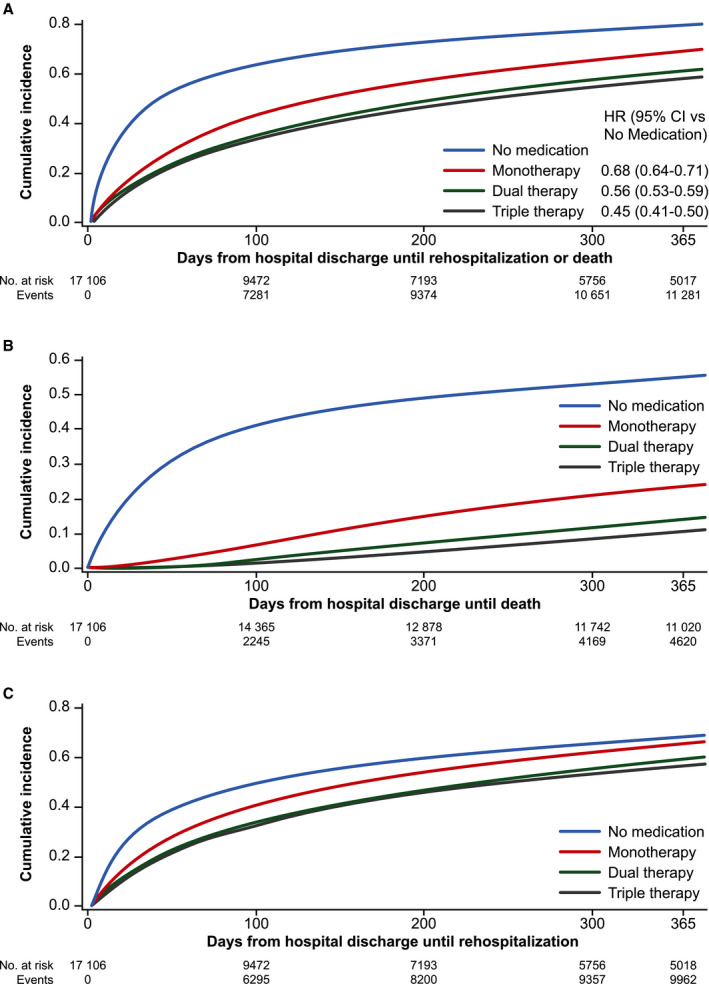
Primary composite outcome: earliest of all‐cause death or all‐cause rehospitalization. No medication: no angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitors, beta‐blockers, or mineralocorticoid receptor antagonist; monotherapy: angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitors or beta‐blockers or mineralocorticoid receptor antagonist; dual therapy: angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitors+beta‐blockers or mineralocorticoid receptor antagonist+beta‐blockers or angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitors+mineralocorticoid receptor antagonist; triple therapy beta‐blockers+angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitors+mineralocorticoid receptor antagonist. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor‐neprilysin inhibitors; BB, beta‐blockers; CI, confidence interval; HR, hazard ratio; MRA, mineralocorticoid receptor antagonist.
Table 2.
Healthcare Resource Utilization Following HF Hospitalization Among Patients With HFrEF
|
No Medication (n=3987) |
Monotherapy (n=3777) |
Dual Therapy (n=7056) |
Triple Therapy (n=2286) |
|
|---|---|---|---|---|
| Deaths, n (%) | 2231 (56.0) | 1049 (27.8) | 1104 (15.6) | 236 (10.3) |
| Mortality PPY (SD) | 20.5 (60.0) | 1.1 (2.9) | 0.4 (2.0) | 0.2 (1.1) |
| Rehospitalizations, n (%) | 2104 (52.8) | 2393 (63.4) | 4161 (59.0) | 1304 (57.0) |
| Incidence PPY (SD) | 4.9 (10.8) | 2.4 (4.9) | 1.7 (2.8) | 1.5 (2.4) |
| Mean length of stay, d (SD) | 7.7 (9.2) | 6.6 (6.7) | 5.8 (5.8) | 5.4 (4.8) |
| ED visits, n (%) | 1501 (37.6) | 1895 (50.2) | 3464 (49.1) | 1164 (50.9) |
| Incidence PPY (SD) | 4.4 (19.7) | 2.2 (4.4) | 1.8 (5.3) | 1.6 (3.0) |
| Observation stays, n (%) | 903 (22.6) | 1120 (29.7) | 2124 (30.1) | 723 (31.6) |
| Incidence PPY (SD) | 4.4 (29.9) | 1.2 (3.7) | 1.0 (3.1) | 1.0 (5.3) |
Column headings denote HF medication use in the year post‐discharge. No medication: no ACEI/ARB/ARNI, BB, or MRA; monotherapy: ACEI/ARB/ARNI or BB or MRA; dual therapy: (ACEI/ARB/ARNI+BB) or (MRA+BB) or (ACEI/ARB/ARNI+MRA); triple therapy (BB+ACEI/ARB/ARNI+MRA). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta‐blocker; ED, emergency department; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; MRA, mineralocorticoid receptor antagonist; and PPY, per person‐year.
Medication Use
Among all patients prescribed post‐discharge medication (n=13 119), 90% received BBs, 74% received ACEIs, ARBs, or ARNIs, and 25% received MRAs (Table 3). Almost half (46%) of patients who received HF medication had no post‐discharge dose escalation; this appeared to decrease with progressive treatment intensity (monotherapy, 63%; dual therapy, 42%; triple therapy, 29%). The proportion of patients dispensed ≥75% of target dose was broadly comparable across treatment intensities (monotherapy, 10%; dual therapy, 12%; triple therapy, 11%). PCPs were responsible for approximately half (51%) of all initial post‐discharge prescriptions, and 20% were prescribed by a cardiologist (Figure 2). Compared with monotherapy and dual therapy, triple therapy was more likely to be prescribed by a cardiologist, and less likely to be prescribed by a PCP.
Table 3.
Medication Use Following HF Hospitalization Among Patients With HFrEF
|
Monotherapy n=3777 |
Dual Therapy n=7056 |
Triple Therapy n=2286 |
|
|---|---|---|---|
| Medications by class, n (%) | |||
| ACEIs, ARBs, or ARNIs | 1012 (26.8) | 6394 (90.6) | 2286 (100.0) |
| BBs | 2598 (68.8) | 6879 (97.5) | 2286 (100.0) |
| MRAs | 167 (4.4) | 839 (11.9) | 2286 (100.0) |
| No dose escalation, n (%) | 2365 (62.6) | 2952 (41.8) | 665 (29.1) |
| Maintenance dose, n (%) | |||
| No. of patients who achieve maintenance dose | 2468 (65.3) | 6129 (86.9) | 2120 (92.7) |
| Maintenance dose 50%–74% of target* | 562 (22.8) | 1528 (24.9) | 692 (32.6) |
| Maintenance dose ≥75% of target* | 254 (10.3) | 745 (12.2) | 242 (11.4) |
Column headings denote HF medication use in the year post‐discharge. Monotherapy: ACEI/ARB/ARNI or BB or MRA; dual therapy: (ACEI/ARB/ARNI+BB) or (MRA+BB) or (ACEI/ARB/ARNI+MRA); triple therapy (BB+ACEI/ARB/ARNI+MRA). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitors; BB, beta‐blockers; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; and MRA, mineralocorticoid receptor antagonist.
Based on target dose recommendations in American College of Cardiology Foundation/American Heart Association guidelines.
Figure 2. Specialty of prescriber.
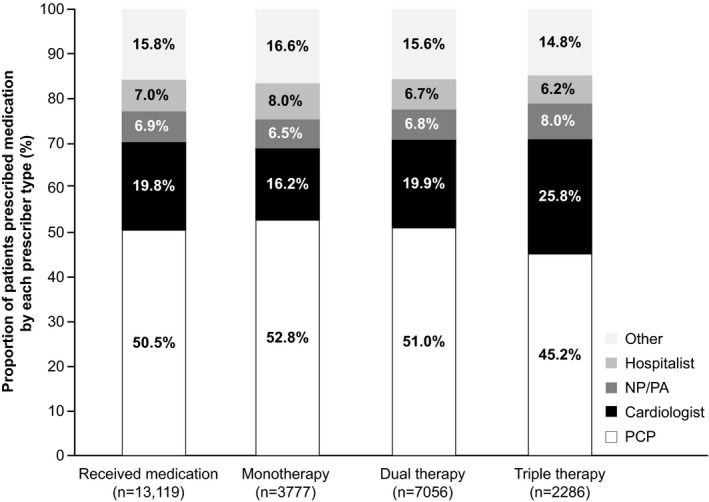
Monotherapy: angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitors or beta‐blockers or mineralocorticoid receptor antagonist; dual therapy: (angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitors+beta‐blockers) or (mineralocorticoid receptor antagonist+beta‐blockers) or (angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitors+mineralocorticoid receptor antagonist); triple therapy: (beta‐blockers+angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitors+mineralocorticoid receptor antagonist). Includes all encounters; not restricted to physician office visits. Specialty categories reflect the first prescription relating to the highest treatment intensity (monotherapy, dual therapy, or triple therapy) reached in the year after hospitalization. “Other” category includes >50 additional provider classifications, with none prescribing to >0.3% of patients who received medication. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor‐neprilysin inhibitors; BB, beta‐blockers; MRA, mineralocorticoid receptor antagonist; NP, nurse practitioner; PA, physician’s assistant; PCP, primary care physician.
Follow‐Up Care
Patients who received no HF medication had proportionately fewer PCP encounters within 14 days (52%) of hospital discharge than patients prescribed monotherapy (61%), dual therapy (61%), or triple therapy (61%) (Figure 3). After 30 days, ≈40% of patients who received no post‐discharge HF medication, and ≈25% of patients prescribed medication, had not received PCP follow‐up. Follow‐up with a cardiologist showed a similar pattern, with proportionately fewer patients having post‐discharge follow‐up in the no HF medication group (21%) than with monotherapy (23%), dual therapy (24%), or triple therapy (26%). Follow‐up rates did not appear to vary substantially with treatment intensity.
Figure 3. Post‐discharge follow‐up within 7, 14, and 30 days with (A) primary care physician; (B) cardiologist.
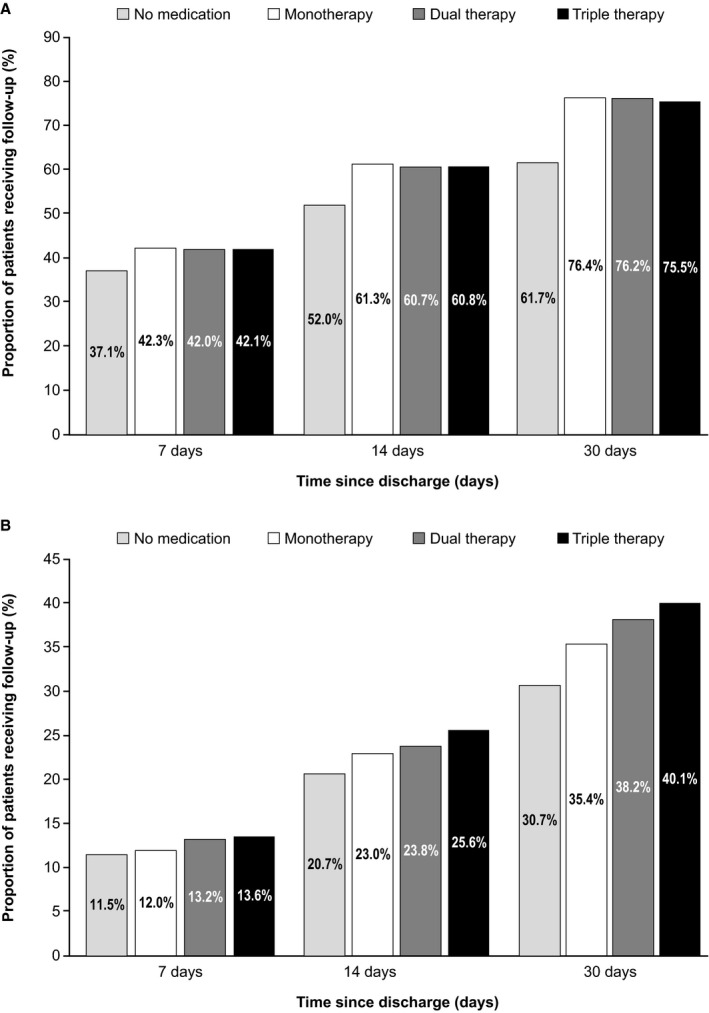
Monotherapy: angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitors or beta‐blockers or mineralocorticoid receptor antagonist; dual therapy: (angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitors+beta‐blockers) or (mineralocorticoid receptor antagonist+beta‐blockers) or (angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitors+mineralocorticoid receptor antagonist); triple therapy: (beta‐blockers+angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitors+mineralocorticoid receptor antagonist). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor‐neprilysin inhibitors; BB, beta‐blockers; MRA, mineralocorticoid receptor antagonist; PCP, primary care physician.
DISCUSSION
In this retrospective observational study of patients with newly diagnosed HFrEF with incident HF‐related hospitalization, we found that higher treatment intensity was associated with improved mortality and rehospitalization risk. This impact of treatment intensity on clinical outcomes reflects previous observations, 17 and prompt initiation of medication before discharge has been shown to improve adherence and survival outcomes. 18 , 19 , 20 The increased incidence of mortality and rehospitalization was particularly apparent during the first 100 days after discharge, which reflects the established 2‐ to 3‐month “vulnerable phase“ immediately following discharge, during which patients are at increased risk of death or clinical events, 21 or increased in risk of rehospitalization. 22 Based on the evidence from this study, a substantial proportion of patients with incident hospitalization for HFrEF have an opportunity to improve treatment in line with guideline recommendations.
Notably, almost a quarter of patients in this study received no ACEI/ARB/ARNI, BB, or MRA in the year after hospitalization per claims data, and almost half did not receive dual or triple therapy as recommended by American College of Cardiology Foundation/American Heart Association guidelines. 1 , 10 Suboptimal rates of initiation of HF‐related pharmacotherapy after HF diagnosis have been reported in previous retrospective analyses of Medicare‐linked data. 5 , 23 Our findings extend these observations by considering treatment intensity and confirm that medication usage rates in patients with HFrEF remain suboptimal even after hospitalization.
Other aspects of follow‐up care after hospitalization for HF also failed to adhere to American College of Cardiology Foundation/American Heart Association guidelines. 1 , 10 Hospitalization did not serve as a triggering event for optimizing HF medication use or dose. This is pertinent, as patients who do not receive medication after discharge have a substantially increased risk of death compared with patients who initiate or continue medication. 24 Post‐discharge uptitration was limited, and few patients (≈10%) reached at least 75% of target dose. Previous observational research has estimated that 74.7% of patients had no treatment modification within 15 days of their first HF‐related hospitalization. 5
These findings are in line with the results of the CHAMP‐HF (Change the Management of Patients With Heart Failure) registry study, which examined the treatment patterns of >3000 US patients in the outpatient setting and found that few patients received target doses of ACEI/ARB (17%), ARNI (14%), and BB (28%). 11 A greater proportion of patients (77%) received target doses of MRAs because MRAs are often initiated at maintenance or near maintenance doses, minimizing the need for titration. It could be argued that physicians submitting data to an observational registry such as CHAMP‐HF were aware of their treatment practices being monitored, and therefore may be more inclined to follow best practice. However, even under these conditions, follow‐up medical care in patients hospitalized for HF remained suboptimal. These data suggest that enhanced coordination of care and improved titration management may be needed.
Further opportunities to improve adherence to guideline recommendations were apparent when assessing post‐discharge follow‐up. Despite evidence that patients who obtain follow‐up within 14 days of hospital discharge have improved clinical outcomes, 25 , 26 only 50% to 60% of patients had a PCP follow‐up within this timeframe, and 20% to 25% had follow‐up with a cardiologist. Even allowing for “lag,” over a quarter of patients had no encounters with their PCP within 30 days, and approximately one third had an encounter with a cardiologist. While transitional care supporting early outpatient follow‐up is essential, it may also be warranted to bolster specialty care in the year post‐discharge. Our study suggests cardiologists appeared more likely to prescribe dual and triple therapy. It is possible, however, that cardiologists see patients with greater severity of illness where dual and triple therapy may be more appropriate.
Adherence to guideline recommendations improves outcomes. 17 , 27 , 28 Randomized controlled clinical trials have established the benefits of reduced morbidity and mortality for ACEI/ARB/ARNI, BB, and MRA therapies in patients with HFrEF. A recent network meta‐analysis of 57 randomized controlled trials compared efficacy of HF medications in patients with HFrEF. 29 That study reported that dual and triple therapy (as per the definitions used in our study) were associated with 43% to 53% and 56% to 63% reductions in mortality compared with placebo, respectively. These observations are consistent with the findings of our real‐world study, and reinforce an important role for increasing treatment intensity in improving clinical outcomes in patients with HFrEF. ACEI/ARB/ARNI, BB, and MRA medications are foundational background therapies for the management of HFrEF. It remains crucial that patients receive early follow‐up post‐discharge where HF medications are appraised and optimized. Reasons for non‐adherence to guidelines are multifactorial. There may be reluctance from providers to initiate or uptitrate medications because of actual or perceived tolerability concerns with HF medications such as BBs, challenges inherent in managing comorbidities and polypharmacy, and a lack of communication between healthcare practitioners in a non‐integrated system. 30 , 31 Additionally, patients may have difficulty complying with guideline directed care because of barriers related to social determinants of health such as food insecurity, transportation challenges, social isolation, and lack of caregiver support. Ongoing, registry‐linked quality improvement initiatives such as OPTIMIZE‐HF (Organized Program To Initiate Lifesaving Treatment In Hospitalized Patients With Heart Failure) and Get With The Guidelines have improved guideline adherence and clinical outcomes in participating centers, 17 , 27 though suboptimal clinical care remains an issue. The consequences of suboptimal care are substantial and as a result efforts to coordinate care for complex patients, including case management and innovations designed to address the challenges faced by patients, are being implemented and evaluated. This is critical since we observed that incidence of rehospitalization and ED visits were higher in patients who received monotherapy or no medication than in patients prescribed dual or triple therapy, and length of stay during rehospitalization was longer.
Prior studies on HF treatment patterns and outcomes have been largely limited to quality improvement registries or clinical trial databases, 1 , 11 , 15 , 23 both of which may be subject to the “Hawthorne effect”, whereby patients and/or physicians may alter their behavior when they are aware of being observed. Using claims data from a large Medicare Advantage plan avoids this effect providing insight into real‐world clinical management of HF in a broad cohort of patients. Additional benefits of this approach include the use of claims data to identify real‐world medication fill patterns; robust assessment of clinical outcomes through comprehensive capture of hospitalization and other patient data; and a level of consistent clinical management enjoyed by patients enrolled in a health plan that may not be present among patients without health coverage.
The current study also has potential limitations. Residual or unmeasured confounding can occur with all observational studies, particularly with the use of claims data. Clinical variables such as HF stage or echocardiogram‐derived ejection fraction are not captured. Such clinical data may have permitted evaluation of the association between disease severity and medication patterns. Social determinants of health are not readily available in claims data but may be a significant reason for lack of compliance with guideline recommended therapy. Furthermore, while our analysis used an algorithm previously validated to identify patients with HFrEF, 16 sensitivity is low (11.8%) which may have resulted in misclassification bias. However, this bias is likely small as the specificity (97.1%) and predictive value (76.7%) of the algorithm is high. 16 The algorithm was developed and validated using ICD‐9 codes and we used both ICD‐9 and ICD‐10 codes to identify HFrEF cases in our study. While ICD‐9 to ICD‐10 mapping is straightforward for the algorithm, the approach is not validated. Our approach for identifying patients also does not account for changes in ejection fraction over time. Many generic BBs and ARBs are low cost and patients may choose to pay out of pocket, as such, we may have underestimated medication initiation and adherence. Records indicate that medications were dispensed, but not necessarily that they were taken as prescribed. In addition, the records do not capture medications that were prescribed but not filled. Additionally, medication use and follow‐up care post‐discharge were descriptive and unadjusted; thus, caution with interpretation is warranted. Additionally, we assessed all‐cause rather than HF‐specific mortality and rehospitalization. Owing to the high rates of comorbidity in these patients, approximately half of post‐discharge rehospitalizations are nominally unrelated to HF. 32 In this regard, assessing all‐cause events was a more comprehensive approach, ensuring that improvements in HF‐related outcomes were not negated by increased risk of non‐cardiac‒related outcomes.
In conclusion, for patients with HFrEF who have been hospitalized with HF, the risk of death and rehospitalization remains high. Our results indicate there are clear opportunities to improve clinical outcomes through enhanced medical management for these patients following an incident hospitalization. Given the importance to patient health of improving outcomes in HF, further investigation to identify other potential interventional factors associated with receipt of optimal HF care is warranted.
Sources of Funding
This work was funded by Amgen Inc.
Disclosures
Wirtz, Honarpour, Kurtz, and Globe are employees of Amgen Inc. and hold Amgen stock/stock options. Wirtz also reports stock ownership in Teva Pharmaceutical Industries, Ltd. Sheer, Pasquale, Casebeer, and Simmons are employees of Humana and hold Humana stock/stock options. Pasquale also reports stock ownership in Amgen Inc.
Supporting information
Table S1
Acknowledgments
Analyses were performed by Humana Healthcare Research Inc. Medical writing assistance was provided by Martin Bell, PhD, of Evidence Medical Affairs. The authors also thank Maya Shehayeb, PharmD, of Amgen Inc. for editorial and writing support.
All authors contributed to the interpretation of data and reviewed and approved the final article.
(J Am Heart Assoc. 2020;9:e015042 DOI: 10.1161/JAHA.119.015042.)
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 2. Curtis LH, Whellan DJ, Hammill BG, Hernandez AF, Anstrom KJ, Shea AM, Schulman KA. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med. 2008;168:418–424. [DOI] [PubMed] [Google Scholar]
- 3. Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long‐term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. [DOI] [PubMed] [Google Scholar]
- 4. Roger VL, Weston SA, Redfield MM, Hellermann‐Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community‐based population. JAMA. 2004;292:344–350. [DOI] [PubMed] [Google Scholar]
- 5. Deschaseaux C, McSharry M, Hudson E, Agrawal R, Turner SJ. Treatment initiation patterns, modifications, and medication adherence among newly diagnosed heart failure patients: a retrospective claims database analysis. J Manag Care Spec Pharm. 2016;22:561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang G, Zhang Z, Ayala C, Wall HK, Fang J. Costs of heart failure‐related hospitalizations in patients aged 18 to 64 years. Am J Manag Care. 2010;16:769–776. [PubMed] [Google Scholar]
- 7. Bueno H, Ross JS, Wang Y, Chen J, Vidan MT, Normand SL, Curtis JP, Drye EE, Lichtman JH, Keenan PS, et al. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303:2141–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, Wang Y, Wang Y, Lin Z, Straube BM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30‐day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407–413. [DOI] [PubMed] [Google Scholar]
- 9. Yancy CW, Januzzi JL Jr, Allen LA, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Jessup M, Lindenfeld J, Maddox TM, et al. 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Task Force on expert consensus decision pathways. J Am Coll Cardiol. 2018;71:201–230. [DOI] [PubMed] [Google Scholar]
- 10. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 11. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP‐HF registry. J Am Coll Cardiol. 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 12. Fonarow GC, Abraham WT, Albert NM, Gattis WA, Gheorghiade M, Greenberg B, O'Connor CM, Yancy CW, Young J. Organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE‐HF): rationale and design. Am Heart J. 2004;148:43–51. [DOI] [PubMed] [Google Scholar]
- 13. Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence‐based heart failure therapies on mortality. Am Heart J. 2011;161:1024–1030.e3. [DOI] [PubMed] [Google Scholar]
- 14. Komajda M, Anker SD, Cowie MR, Filippatos GS, Mengelle B, Ponikowski P, Tavazzi L; Investigators Q . Physicians' adherence to guideline‐recommended medications in heart failure with reduced ejection fraction: data from the QUALIFY global survey. Eur J Heart Fail. 2016;18:514–522. [DOI] [PubMed] [Google Scholar]
- 15. Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. [DOI] [PubMed] [Google Scholar]
- 16. Li Q, Glynn RJ, Dreyer NA, Liu J, Mogun H, Setoguchi S. Validity of claims‐based definitions of left ventricular systolic dysfunction in Medicare patients. Pharmacoepidemiol Drug Saf. 2011;20:700–708. [DOI] [PubMed] [Google Scholar]
- 17. Fonarow GC, Albert NM, Curtis AB, Gheorghiade M, Liu Y, Mehra MR, O'Connor CM, Reynolds D, Walsh MN, Yancy CW. Incremental reduction in risk of death associated with use of guideline‐recommended therapies in patients with heart failure: a nested case‐control analysis of IMPROVE HF. J Am Heart Assoc. 2012;1:16–26. DOI: 10.1161/JAHA.111.000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gattis WA, O'Connor CM, Gallup DS, Hasselblad V, Gheorghiade M; Investigators I‐H, Coordinators . Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT‐HF) trial. J Am Coll Cardiol. 2004;43:1534–1541. [DOI] [PubMed] [Google Scholar]
- 19. Gayat E, Arrigo M, Littnerova S, Sato N, Parenica J, Ishihara S, Spinar J, Muller C, Harjola VP, Lassus J, et al. Heart failure oral therapies at discharge are associated with better outcome in acute heart failure: a propensity‐score matched study. Eur J Heart Fail. 2018;20:345–354. [DOI] [PubMed] [Google Scholar]
- 20. Butler J, Arbogast PG, Daugherty J, Jain MK, Ray WA, Griffin MR. Outpatient utilization of angiotensin‐converting enzyme inhibitors among heart failure patients after hospital discharge. J Am Coll Cardiol. 2004;43:2036–2043. [DOI] [PubMed] [Google Scholar]
- 21. Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol. 2015;12:220–229. [DOI] [PubMed] [Google Scholar]
- 22. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013;61:391–403. [DOI] [PubMed] [Google Scholar]
- 23. Albert NM, Drzayich Antol DA, DeClue RW, Casebeer AW, Li Y, Stemkowski S, Chang CL. Pharmacotherapy choice is associated with 2‐year mortality for patients with heart failure and reduced ejection fraction. Adv Ther. 2017;34:2345–2359. [DOI] [PubMed] [Google Scholar]
- 24. Gilstrap LG, Fonarow GC, Desai AS, Liang L, Matsouaka R, DeVore AD, Smith EE, Heidenreich P, Hernandez AF, Yancy CW, et al. Initiation, continuation, or withdrawal of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers and outcomes in patients hospitalized with heart failure with reduced ejection fraction. J Am Heart Assoc. 2017;6:e004675 DOI: 10.1161/JAHA.116.004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Atzema CL, Austin PC, Yu B, Schull MJ, Jackevicius CA, Ivers NM, Rochon PA, Lee DS. Effect of early physician follow‐up on mortality and subsequent hospital admissions after emergency care for heart failure: a retrospective cohort study. CMAJ. 2018;190:E1468–E1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McAlister FA, Youngson E, Kaul P, Ezekowitz JA. Early follow‐up after a heart failure exacerbation: the importance of continuity. Circ Heart Fail. 2016:9:e003194. [DOI] [PubMed] [Google Scholar]
- 27. Heidenreich PA, Lewis WR, LaBresh KA, Schwamm LH, Fonarow GC. Hospital performance recognition with the Get With The Guidelines Program and mortality for acute myocardial infarction and heart failure. Am Heart J. 2009;158:546–553. [DOI] [PubMed] [Google Scholar]
- 28. Komajda M, Cowie MR, Tavazzi L, Ponikowski P, Anker SD, Filippatos GS; Investigators Q . Physicians' guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail. 2017;19:1414–1423. [DOI] [PubMed] [Google Scholar]
- 29. Burnett H, Earley A, Voors AA, Senni M, McMurray JJ, Deschaseaux C, Cope S. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction: a network meta‐analysis. Circ Heart Fail. 2017;10:e003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chioncel O, Collins SP, Ambrosy AP, Pang PS, Antohi EL, Iliescu VA, Maggioni AP, Butler J, Mebazaa A. Improving postdischarge outcomes in acute heart failure. Am J Ther. 2018;25:e475–e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gilstrap LG, Stevenson LW, Small R, Parambi R, Hamershock R, Greenberg J, Carr C, Ghazinouri R, Rathman L, Han E, et al. Reasons for guideline nonadherence at heart failure discharge. J Am Heart Assoc. 2018;7:e008789 DOI: 10.1161/JAHA.118.008789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maggioni AP, Orso F, Calabria S, Rossi E, Cinconze E, Baldasseroni S, Martini N; ARNO Observatory . The real‐world evidence of heart failure: findings from 41 413 patients of the ARNO database. Eur J Heart Fail. 2016;18:402–410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


