Figure 3. Trial sequential analysis for MACE (A) and clinically significant bleeding (B) end points.
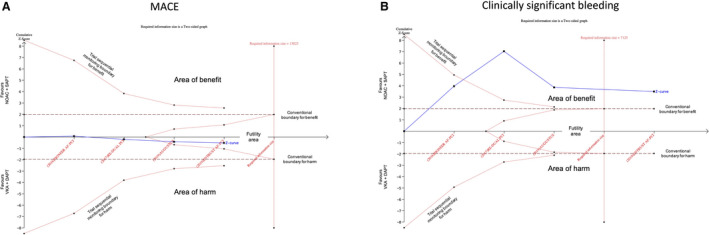
The vertical red dotted line represents required information size (ie, the number of patients required) to definitely demonstrate the risk difference (alpha 5%, power 80%). The horizontal axis represents the number of patients included in the meta‐analysis and is linear scaled, hence the distance of a new trial from the previous one on the axis represents the new trial population. The vertical axis represents the cumulative z‐score. The red dotted lines represent the trial sequential monitoring boundaries (inward sloping) and the futility boundaries (outward sloping). The solid blue line represents the cumulative z‐curve. According to the trial sequential analysis methodology, crossing the monitoring boundaries for the z‐curve indicates a clinically meaningful effect of a specific intervention that is also supported by statistical significance; crossing the required information size line indicates that the evidence is conclusive, whereas being in the futility area suggest that the effect size is neither clinically nor statistically meaningful and it is improbable that with further trials the cumulative evidence could demonstrate a significance in the effect size. In panel A, the required information size to demonstrate or reject a 35% relative risk reduction with an incidence in the control group of 22.6% is 7125 patients (required information size line). With the ENTRUST AF‐PCI trial the z‐curve crossed the required information size line. In panel B, the required information size to demonstrate or reject a 20% relative risk reduction with an incidence in the control group of 7% is 13 023 patients. With the AUGUSTUS and ENTRUST AF‐PCI trial the z‐curve entered the futility area. AUGUSTUS indicates A Study of Apixaban in Patients With Atrial Fibrillation, Not Caused by a Heart Valve Problem, Who Are at Risk for Thrombosis (Blood Clots) Due to Having Had a Recent Coronary Event, Such as a Heart Attack or a Procedure to Open the Vessels of the Heart; DAPT, dual antiplatelet therapy; ENTRUST‐AF PCI, Edoxaban Treatment vs Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention; MACE, major adverse cardiovascular event; NOAC, non–vitamin K antagonist oral anticoagulant; SAPT, single antiplatelet therapy; and VKA, vitamin K antagonist.
