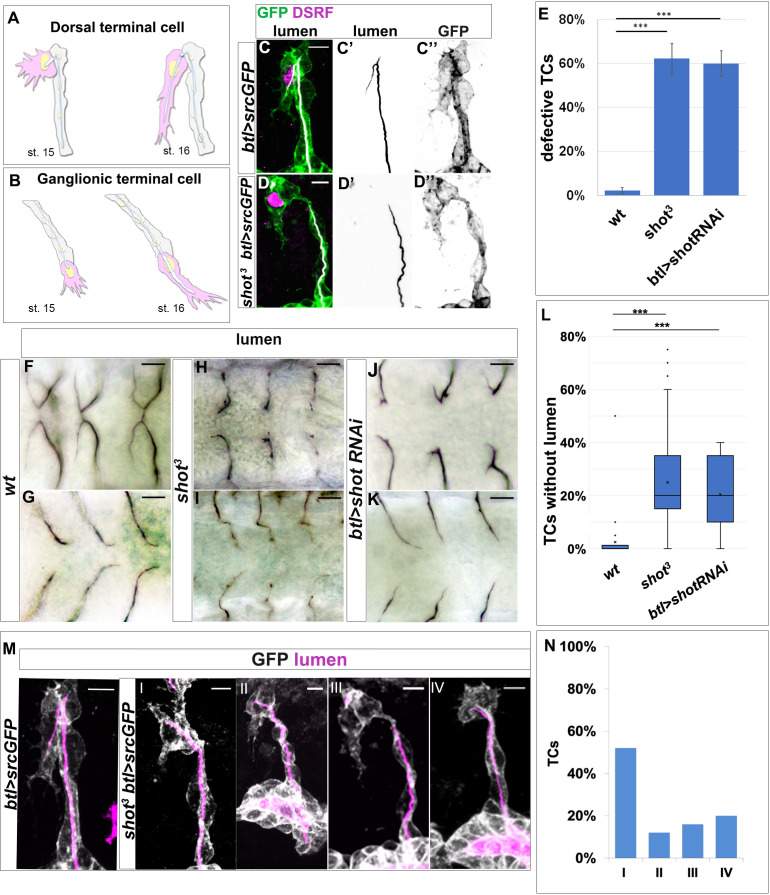
Figure 1. shot loss-of-function induces defects in subcellular lumen formation.
(A–B) Representation of dorsal and ganglionic TCs from embryonic st.15 to st.16 (DB and GB in grey, TC in pink). At st.15, the TC (cytoplasm in pink, nucleus in yellow, basal membrane in grey, apical membrane in blue and lumen in white) emits filopodia in the direction of cell elongation; apical membrane grows in the same direction giving rise to the outline of the subcellular lumen. At the same time the subcellular lumen is filled of chitin (white). At the end of st.16 the TC is elongated and the subcellular lumen is formed. (C–D) DBs at st.15 of btl >srcGFP (control) and shot3; btl >srcGFP fixed embryos stained with GFP to visualise tracheal cells, green in C and D, grey in C’’ and D’’, CBP to visualise the lumen, white in C and D black in C’ and D’ and DSRF in magenta. Anterior side is on the left and dorsal is up, scale bars 5 μm. (E) Quantification of total defective TCs in btl >shotRNAi (60%), shot3 (62.5%) and wt (2.25%) n = 20 embryos, 400TCs. Error bars are ± SEM and asterisks represent a p-value<0001. Statistics by two-tailed Student’s t-test. (F–K) DBs (F-J dorsal view) and GBs (G-K ventral view) of fixed embryos stained with anti-Gasp antibody at st.16 of wt (F and G), shot3 (H and I) and btl >shotRNAi (J and K) (L) Quantification of total TCs (genotype indicated) without subcellular lumen (wt 1.34% n = 400, shot3 25% n = 400, btl >shotRNAi 20%n = 300). *** p-value<0001. Statistics by two-tailed Student’s t-test. Scale bars 10 µm. (M–N) Different types of TC mutant phenotypes were produced in absence of Shot as observed in detail by confocal microscopy. (M) Dorsal branches of btl >srcGFP control and shot3 embryos stained with GFP (grey) to visualise membrane and CBP (in magenta) to visualise the lumen. Anterior side is on the left and dorsal side is up. Scale bars 5 μm. (I) TC partially elongated with formed lumen but with wrong directionality (52%); (II) the elongation was stopped prematurely and a primordium of subcellular lumen was formed (12%); (III) the cell elongated partially but the lumen was completely absent (16%); and (IV) the cell was not able to elongate and the lumen was completely absent (20%). Types III and IV were quantified in L as TCs without lumen. (E) Detailed quantification, by confocal microscopy, of the different types of TC mutant phenotypes reported as I-IV (n = 25 TCs).