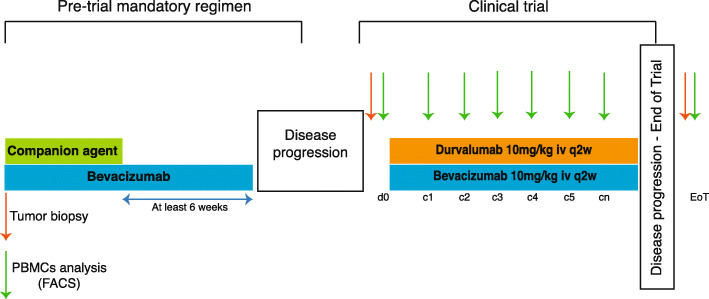
Fig. 1.
Trial design. Patients had to be receiving maintenance treatment with bevacizumab alone as a part of a previous bevacizumab-containing regimen for advanced disease, after discontinuing the companion agent because of the usual clinical practice reasons (cumulative toxicity or achievement of maximal disease response). Pre-trial bevacizumab maintenance was allowed at 5 mg/kg weekly, 10 mg/kg q2w, or 15 mg/kg q3w. When patients experienced disease progression while on bevacizumab treatment become candidates for the trial. Bevacizumab treatment was never stopped, and the first durvalumab dose was scheduled for infusion on the next planned bevacizumab dose. All patients switched to a 10 mg/kg weekly bevacizumab schedule in case they were receiving it on a different one. A fresh tumor biopsy was obtained within a time-window of 7 days prior to the first durvalumab dose. In addition, a PBMC sample was obtained on day 1 prior to the first durvalumab dose, and repeated periodically until disease progression; an additional sample was harvested at the end-of-treatment visit (28 days after coming-off trial). Treatment continued until disease progression, unacceptable toxicity or investigator decision