Fig. 2.
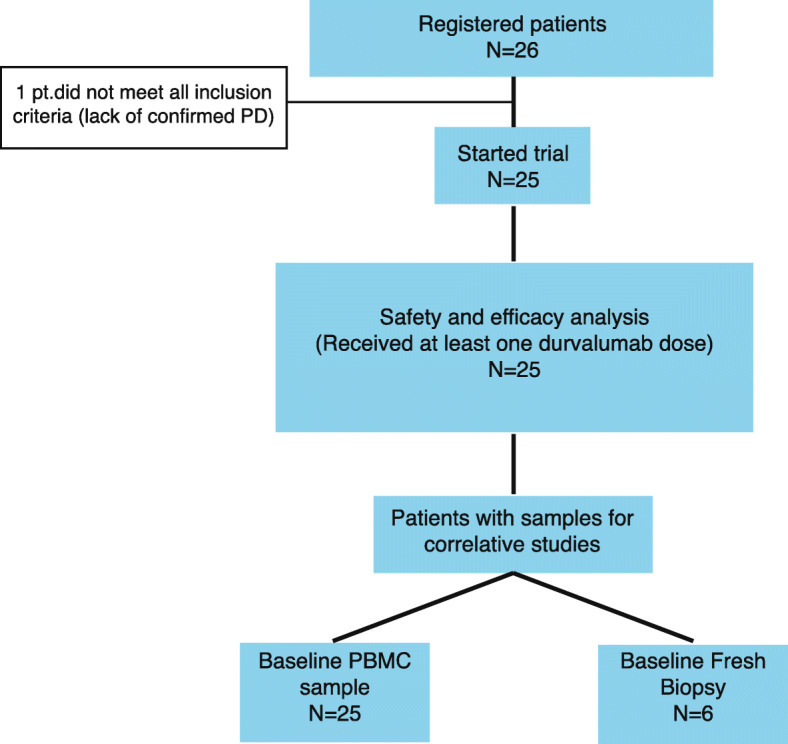
CONSORT diagram. Twenty six patients underwent trial screening but one patient was deemed ineligible since she was not found to have documented progressive disease to ongoing bevacizumab maintenance. The rest of the patients (N = 25) received at least 1 durvalumab dose and were included in the safety and efficacy analysis. All patients had at least one baseline PBMCs sample for the immune subpopulation analysis; of them, only 6 consented for the pre-treatment tumor biopsy that was subsequently used for immunohistochemistry and gene-expression studies
