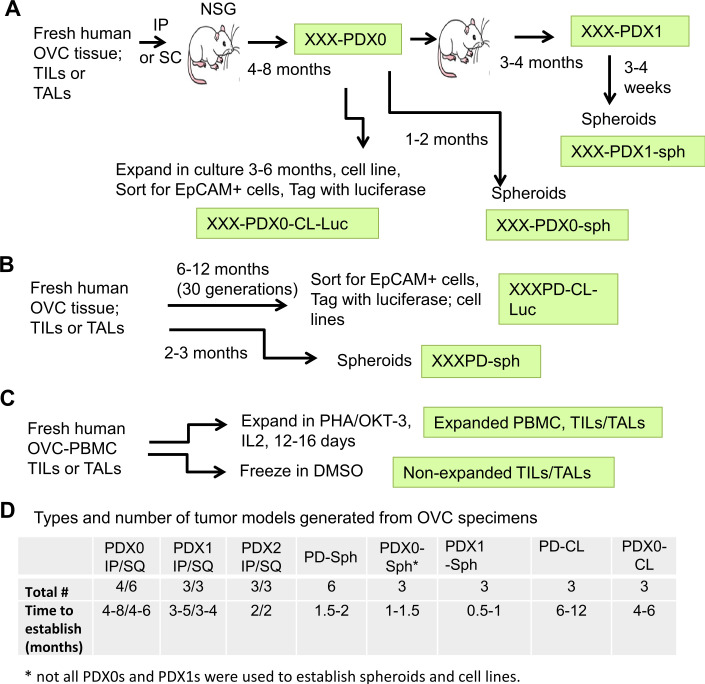
Figure 1.
Overview of workflow for generating OVC PDXs in NSG mice, spheroids and cell lines, and expansion of TILs and TALs. (A) Establishment of PDXs. Fresh ovarian solid tumors or ascites fluid were made single cell or small clumps and injected intraperitoneally (IP) or subcutaneously (SQ) into NSG mice to generate PDXs. Tumors formed from the patients’ specimens were designated as PDX0. XXX represents tumor identification number. The PDX0 tumors were minced and reimplanted into tumor-naive NSG mice for next passage and were designated as Pdx1. Some of the PDXs were also expanded in vitro to generate spheroids (PDX0-sph) or cell lines (PDX0-CL) as described below. (B) Establishment of spheroids or cell lines. Cell suspension from the fresh human specimens or PDXs and their derivatives were cultured in spheroid growth medium or RMPI1640 complete medium to obtain spheroids (PD-sph) or cell lines (PD-CL), respectively. Some cell lines were sorted for epithelia phenotype with EpCAM antibody and tagged with luciferase for bioluminescence imaging. (C) Expanding autologous TILs/TALs. Single cells and/or small cell clumps were expanded using PHA (phytohaemagglutinin, 1 µg/mL) and OKT3 (1 µg/mL) in the presence of IL2 for 12–16 days or were frozen in 10% dimethyl sulfoxide (DMSO, non-expanded TILs/TALs). (D) Summary of types and number of tumor models generated from OVC specimens in this study. Average time observed in establishing the models is shown. IL, interleukin; IP, intraperitoneally; OVC, ovarian cancer; PBMC, peripheral blood mononuclear cell; PDX0-CL, patient-derived cell lines; PDX0-sph, patient-derived spheroid lines; SQ, subcutaneously; TALs, tumor-associated leukocytes; TIL, tumor-infiltrating leukocyte.