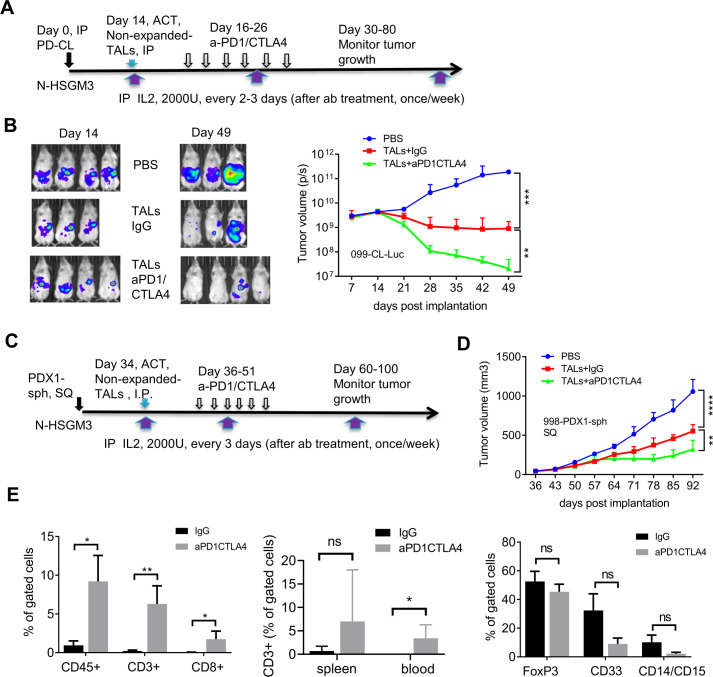
Figure 5.
Adoptive transfer of non-expanded autologous TALs in combination with PD-1/CTLA4 blockade significantly inhibit the growth of IP-injected 099-CL-Luc and SQ-injected 998-PDX1-sph in N-HSGM3 mice. (A) Schema of 099-CL-Luc tumor implantation, ACT and ICB treatment. Fourteen days after tumor implantation, mice (n=3–4) were imaged and treated intraperitoneally with IgG or blockade antibodies every other day for six times. (B) Tumor load was assessed by bioluminescent imaging of 099-CL-Luc OVC tumor-bearing mice at indicated time points post implantation. Tumor volume data are presented as mean±SEM, and are from one of the duplicated experiments. (C) Schema of 998-PDX1-sph tumor implantation, ACT and ICB treatment. (D) Tumor growth of 998-PDX1-sph was assessed using a caliper at indicated time points postimplantation. Tumor volume data are presented as mean±SEM from one of the two independent experiments. (E) Dual ICB increased CD3+CD8+ T cells and decreased CD33+ MDSC cells in the peritoneal wash (PW) Tme. Left panel, frequencies of CD45+, CD3+ and CD8+ T cells were all significantly higher in the aPD1/CTLA4-treated PW than the IgG-treated group. Middle panel, frequency of CD3+ T cells in the spleen and blood samples. Right panel, frequency of FOXP3 and MDSC populations in PW samples. Live cells from the peritoneal wash, spleen and blood samples from the IgG- and dual aPD1/CTLA4-treated mice were stained with antibodies specific for CD45, CD3, and CD8 for T cells or CD4, CD25, and FOXP3 for T regulatory cells, or CD45, CD33, CD11b, CD14, and CD15 for MDSC cells. *P<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns, not significant, using two-way ANOVA (B, D) or unpaired two-tail t-test (E). ACT, adoptive cell transfer; ANOVA, analysis of variance; ICB, immune checkpoint blockers; IL, interleukin; IP, intraperitoneally; MDSC, myeloid-derived suppressive cells; OVC, ovarian cancer; PBMC, peripheral blood mononuclear cell; SQ, subcutaneously; TALs, tumor-associated leucocytes; TILs, tumor-infiltrating leukocytes.