Abstract
A 46-year-old man with Klinefelter syndrome (KS) presented with obliterative phlebitis of the lower legs with a deteriorated renal function, and elevated serum alkaline phosphatase and ataxia levels. Examinations demonstrated tubulointerstitial nephritis, obliterative phlebitis and lymphadenopathy with IgG4+ plasma cell infiltrate and sclerosing cholangitis. Although the serological profile and central nerve system involvement were compatible for systemic lupus erythematosus (SLE), a definite diagnosis of SLE was difficult to make. IgG4-related disease (IgG4-RD) with KS was finally diagnosed, and high dose prednisolone with intravenous cyclophosphamide was initiated and thereafter the patient demonstrated a prompt improvement. This is the first known case demonstrating overlapping IgG4-RD with lupus-like serological and neurological features in a patient with KS, thus highlighting the pathogenic role with the genomic background for IgG4-RD and SLE.
Keywords: Klinefelter syndrome, IgG4-related disease, systemic lupus erythematosus, chromosomal disorder, autoimmune disease
Introduction
Klinefelter syndrome (KS) is a chromosomal disorder affecting males and it is characterized by the presence of an extra X chromosome generating a 47, XXY karyotype (1). The extra X chromosome is responsible for its specific clinical features, such as sex hormone imbalance, metabolic disorders, absence of secondary sexual characteristics, and sterility (1, 2). People with KS are at an increased risk of developing diseases that include certain cancers, cardiovascular diseases, and autoimmune diseases (3, 4). Systemic lupus erythematosus (SLE) is an autoimmune, connective tissue disease characterized by the formation of multiple autoantibodies and immune complexes resulting in systemic organ involvement (5). Women of reproductive age are affected 10 times more frequently than men. A high risk of SLE complicating KS has been reported previously (3, 4, 6, 7).
IgG4-related disease (IgG4-RD) is an immune-mediated systemic disease of an unknown aetiology recognized recently. It is characterized by elevated serum IgG4 levels, infiltration of IgG4-positive plasma cells, obliterative phlebitis, and tissue fibrosis of the affected organs (8). Its association with autoimmune diseases such as SLE (9-12), Sjögren syndrome (13, 14), antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (15), and relapsing polychondritis (16) is suggestive of a possible similarity in the immune-disturbances observed in these diseases. We herein report an interesting overlapping case of IgG4-RD and KS with lupus-like serological and neurological features.
Case Report
A 46-year-old Japanese man with KS (46XY/47XXY mosaic) was admitted to Keio University Hospital with bilateral pain and swelling in his lower legs and a skin ulcer on his left ankle in July 2010. Biopsy from the ulcerative skin lesion revealed obliterative phlebitis. Although antinuclear antibody was positive, SLE-specific autoantibodies were negative and no clinical features suggesting SLE were observed. He was treated effectively with a high dose of prednisolone (PSL) of 60 mg/day. There was relapse of the obliterative phlebitis in December 2012. He was administered 3 mg/day of PSL; increasing the dose to 30 mg/day aided in resolving the inflammation. In October 2015, he complained of an unstable gait. He was diagnosed of cerebellar ataxia of unknown aetiology, despite extensive examinations. He subsequently manifested mild behavioral abnormalities such as obsessive tendencies along with the emergence of lymphopenia. Following a reduction of the dose of PSL to 3 mg/day in August 2018, a relapse of obliterative phlebitis with skin ulceration, deteriorated renal function, and elevated serum alkaline phosphatase (ALP) levels were observed. He was subsequently admitted to the hospital.
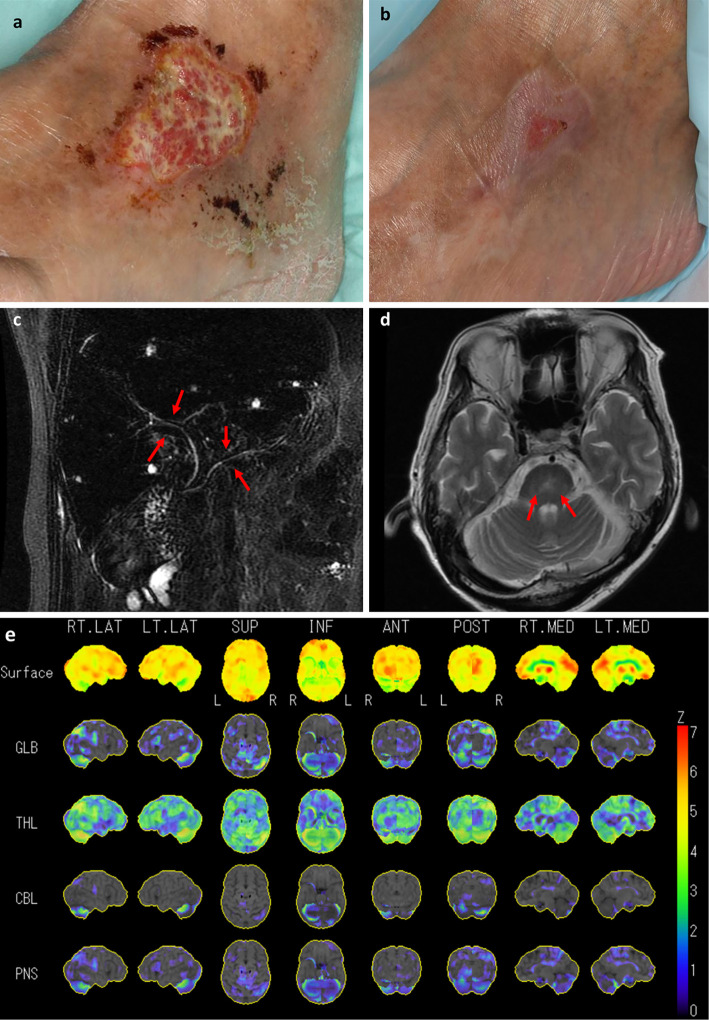
While his vital signs were normal, physical examination revealed gynecomastia, lymphadenopathy of the neck, and an ulcer on the left leg (Fig. 1a). Neurological examinations revealed ataxia of the left side, and its severity was deemed grade 2 according to the scale for the assessment and rating of ataxia (17). The results of laboratory investigations are shown in Table 1. A contrast enhanced computed tomographic scan revealed multiple enlarged lymph nodes of the neck, supraclavicular fossa, axilla, para-aorta, obturator and inguinal. Magnetic resonance cholangiopancreatography (Fig. 1c) and endoscopic ultrasonography demonstrated a narrowing of the common bile duct suggestive of sclerosing cholangitis. Magnetic resonance imaging of the brain showed an abnormal T2 enhancement in the central pons and the cerebellum (Fig. 1d). Single photon emission computed tomography revealed a decreased blood flow in the cerebral hemisphere bilaterally (Fig. 1e). Electroencephalography demonstrated globally synchronous occasional slow waves.
Figure 1.
A skin lesion of obliterative phlebitis at ankle and magnetic resonance imaging of the abdomen and brain. (a) At the time of admission in 2018 (b) 2 months after treatment. (c) Magnetic resonance cholangiopancreatography revealed a narrowing of common bile duct. (d) brain magnetic resonance imaging revealed abnormal T2 enhancement in the central pons to cerebellum. (e) single photon emission computed tomography revealed a decreased blood flow in the cerebral hemisphere bilaterally.
Table 1.
Laboratory Findings of This Patient.
| Blood test | Normal range | At admission |
|---|---|---|
| White blood count, /μL | 3,500-8,500 | 9,400 |
| Neutrophil, % | 40-70 | 53 |
| Lymphocyte, % | 20-50 | 11.5 |
| Monocyte, % | 2-9 | 9.5 |
| Eosinophil, % | 1-6 | 15.5 |
| Basophil, % | 0-2 | 3.0 |
| Atypical lymphocyte, % | 7.0 | |
| Haemoglobin, g/dL | 13.5-17.0 | 7.4 |
| Platelet,×104/μL | 15.0-35.0 | 27.0 |
| Blood urine nitrogen, mg/dL | 8-20 | 18.2 |
| Creatinine, mg/dL | 0.7-1.1 | 1.2 (elevated from his baseline 0.8 mg/dL) |
| Asparate aminotransferase, U/L | 10-35 | 12 |
| Alanine aminotransferase, U/L | 5-40 | 8 |
| Lactate dehydrogenase, U/L | 120-220 | 215 |
| Alkaline phosphatase, U/L | 100-320 | 1,055 |
| Type 1, % | 0 | 28 |
| Type 2, % | 36-74 | 65 |
| Type 3, % | 25-59 | 7 |
| γ-glutamyl Transpeptidase, U/L | 10-90 | 63 |
| C-reactive proteins, mg/dL | <0.35 | 2.72 |
| IgG, mg/dL | 870-1,700 | 4,727 |
| IgG4, mg/dL | 4.8-105 | 1,826 |
| IgM, mg/dL | 33-190 | 243 |
| IgA, mg/dL | 110-410 | 179 |
| IgE, IU/mL | 0-170 | 3,100 |
| C3,mg/dL | 65-135 | 18 |
| C4, mg/dL | 13-35 | 2 |
| CH-50, U/mL | 32-58 | <10 |
| Immune complex (C1q), μg/mL | <3 | 10.6 |
| Soluble interleukin-2 receptor, U/mL | 142-500 | 6,652 |
| Direct Coombs test | Negative | Positive |
| Anti-nuclear antibody | ||
| pattern | <40 | 1,280 folds |
| Homogeneous, speckled | ||
| Anti-double stranded DNA antibody (enzyme-linked immunosorbent assay), IU/mL | <12 | 24.0 |
| Anti-DNA antibody (radioimmunoassay), IU/mL | <6 | 10 |
| Anti-Sm antibody | Negative | Negative |
| Anti-RNP antibody | Negative | Negative |
| Anti-SS-A antibody | Negative | Negative |
| Anti- SS-B antibody | Negative | Negative |
| Anti-cardiolipin antibody | Negative | Negative |
| Anti-β2 glycoprotein I antibody | Negative | Negative |
| Lupus anticoagulant | Negative | Negative |
| Anti-neutrophil cytoplasmic antibody | Negative | Negative |
| Rheumatoid factor | <15 | 155 |
| Anti-cyclic citrullinated peptide antibody | Negative | Negative |
| Urinalysis | ||
| Proteinuria | Negative | Negative |
| Haematuria | Negative | Negative |
| Cast | Negative | Negative |
| N-acetyl-β-D-glucosaminidase, U/L | <11.5 | 16.7 |
| β2-microglobulin, μg/L | <200 | 8,691 |
| α1-microglobulin, mg/L | <8.3 | 16 |
| Cerebrospinal fluid | ||
| Appearance | Colourless and transparent | |
| Cell count | 0-5 | 3 (mono) |
| Opening pressure, cmH20 | 6-15 | 12 |
| Total protein, mg/dL | 15-45 | 32 |
| Glucose, mg/dL | - | 62 |
| IgG index | 0-0.73 | 0.29 |
| Oligoclonal band | Negative | Negative |
| Interleukin-6, pg/mL | <4.0 | 5.9 |
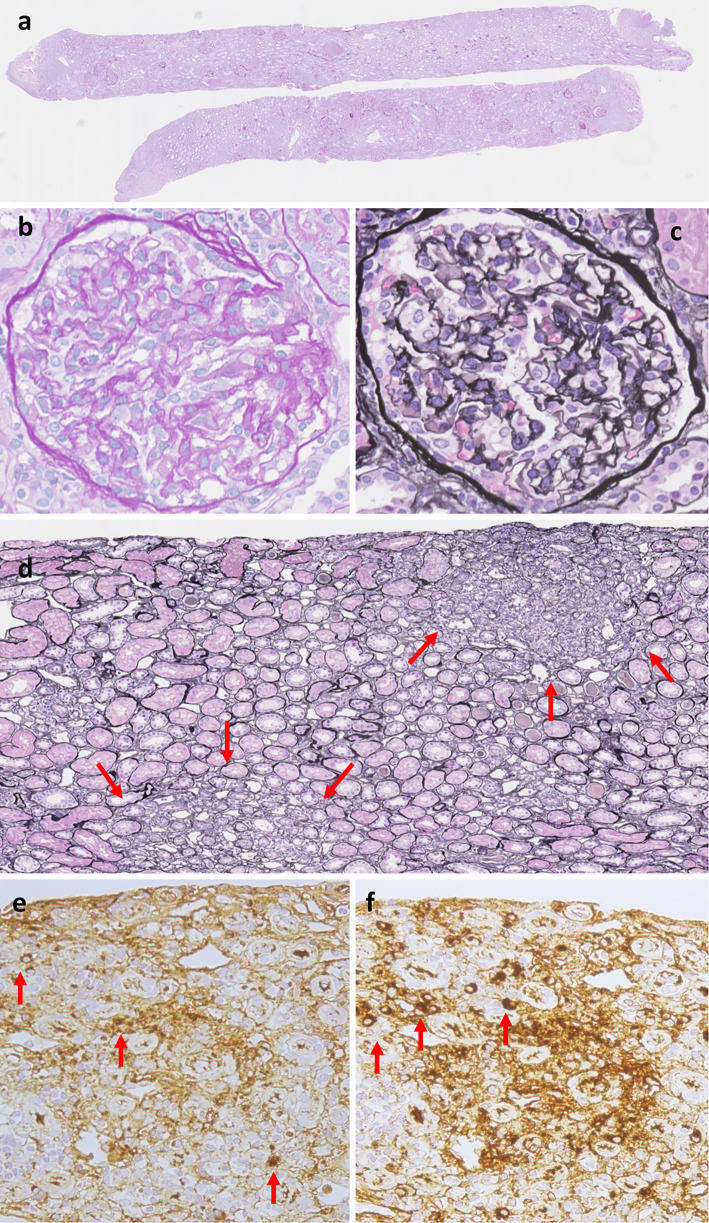
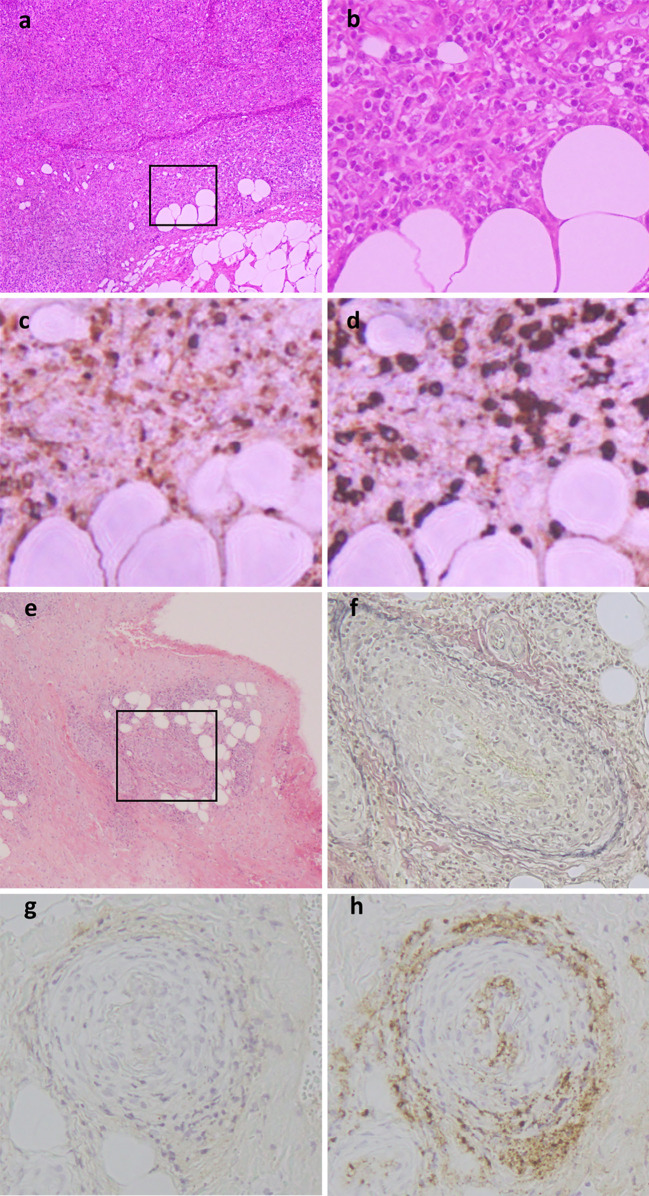
A renal and cervical lymph node biopsy was performed. The renal sections demonstrated segmental interstitial inflammation with fibrosis and infiltration of IgG4 positive plasma cells without glomerular abnormality. The IgG4 positive plasma cells were observed at more than 10 cells/HPF and accounted for 40% of IgG positive cells and (Fig. 2). Immunofluorescence staining showed mesangial deposition of IgG (IgG1 +, IgG2±, IgG3±, IgG4 ++) and IgM. These findings were compatible for IgG4-related tubulointerstitial nephritis. Lymph nodes showed dense lymphoplasmacytic infiltration; immunohistochemical staining showed that more than 40% of the plasma cells were positive for IgG4 (Fig. 3a-d). In addition, a skin specimen obtained in 2010 was re-evaluated by additional staining for IgG and IgG4. There were features suggestive of obliterative phlebitis with an equal distribution of IgG4 and IgG positive plasma cells (Fig. 3e-h). These pathological findings lead to a diagnosis of IgG4-RD (obstructive phlebitis, sclerosing cholangitis, kidney disease) based on the comprehensive diagnostic criteria established in 2011 (18).
Figure 2.
Histological findings of the kidney. (a) Periodic acid-Schiff (PAS) staining at a low power field (×50). Cortex: medulla ratio was 6: 4. This specimen contained 42 glomeruluses, 11 sclerosed and 2 collapsed. (b) PAS staining of glomerulus at a high power field (×200). There was no endocapillary abnormality. (c) periodic acid-methenamine-silver (PAM) staining of glomerulus at a high power field (×200). There was no subepithelial change. (d) PAM staining of interstitium at low power field (×100). There was segmental lymphoplasmacytic infiltration with fibrosis. (e) IgG staining of interstitium at high power field (×200). (f) IgG4 staining of interstitium (×200) at a high power field.
Figure 3.
Histological findings of the lymph node and skin. (a) Hematoxylin and Eosin (H&E) staining at a low power field (×40). Normal structure of lymph follicle with lymphoplasmacytic infiltration was observed, and T cells and B cells are mixed. There was no monoclonal cell proliferation or lymphoproliferative disorder. (b) H&E staining of lymph node specimen at a high power field (×400). (c) IgG staining at a high power field (×400). (d) IgG4 staining at a high power field (×400). IgG4 /IgG ratio was more than 40%. (e) H&E staining of a skin specimen at a low power field (×40). Obliterative phlebitis with lymphoplasmacytic infiltration and eosinophil were observed. (f) Elastica van Gieson staining at a high power field (×200). (g) IgG staining at a high power field (×200). (h) IgG4 staining, a high power field (×200). The number of IgG4 positive plasma cells was the same as the number of IgG positive plasma cells.
On the other hand, the presence of a high titer of antinuclear antibodies and anti-dsDNA antibody, low complement with positive immune complex, a positive direct coombs test, lymphopenia, neuropsychological abnormality (ataxia, obsession, abnormal electroencephalography, and high interleukin (IL)-6 concentrations in the cerebrospinal fluid) were not explained by a diagnosis of IgG4-RD. Although these findings fulfilled the SLE classification criteria established by the American College of Rheumatology in 1997 (19) and 2019 European league Against Rheumatism/American College of Rheumatology classification criteria (20), typical clinical features of SLE were lacking for the definite diagnosis. Thus, his overall final diagnosis was that of an overlapping IgG4-RD with lupus-like serological and neurological features in KS.
PSL of 60 mg/day and monthly intravenous cyclophosphamide (IVCY) of 750 mg/m2 were initiated as induction therapy for recurrent IgG4-RD and lupus-like neuropsychiatric findings. The treatment improved the clinical manifestations of IgG4-RD in the organs, including the skin ulcer (Fig. 1b) and the lymphadenopathy, and led to a rapid improvement in the levels of serum ALP, creatinine, IgG4, sIL-2R, positive anti-dsDNA antibody, low complement and circulating immune complexes. The lupus-like neuropsychiatric findings including ataxia and abnormal behavior reverted to normal. IL-6 in the cerebrospinal fluid also returned to normal levels.
Discussion
An overlapping case of IgG4-RD and KS with lupus-like serological and neurological features is herein presented. This case highlights the contributory role of autoimmune disturbances based on genetic factors in the development of other diseases through varying mechanisms.
KS is a chromosomal disorder of a 47, XXY karyotype (1, 2). The abundance of X chromosomes is known to cause diseases which are prone to occur more frequently in females (4, 9-15). Several national registry-based reports have highlighted a female predilection for SLE with a sex ratio of 9:1 female to male, while the predilection in patients with KS is 18 times higher than that in the general population (3, 4, 6, 7). Genes on one X chromosome are usually inactivated by histone modifications and promoter DNA methylation in order to adjust for the genetic imbalance on the X chromosome amongst males and females. However, a part of X-linked human genes escapes X chromosome inactivation such that both alleles are expressed simultaneously in various cells including immune cells (21). Studies on KS have shown that toll-like receptor 7 (TLR7) escapes X chromosome inactivation in plasmacytoid dendritic cells and B lymphocytes leading to a higher incidence of TLR7-driven responses (22). Furthermore, genome-wide association studies (GWAS) in SLE have enabled the extraction of several genes related to the pathogenesis of SLE, which include genes located on the X chromosome such as TLR7, interleukin-1 receptor-associated kinase 1 (IRAK1), and methyl-CpG-binding protein 2 (MECP2) genes related to type I interferon signaling (23-25). These genes can attribute to an increased risk of SLE in KS. There has been no report showing an association between IgG4-RD and IRAK1 or MECP2, however, a recent report revealed that TLR-7-expressing M2 macrophages promote the activation of Th2 immune responses via IL-33 secretion in IgG4-RD (26).
There are differences in the clinical features of SLE in patients with KS compared to SLE afflicted normal male patients (7). The clinical manifestations in patients with KS are usually mild and rarely include severe organ involvement such as proliferative renal disease, neurological disease, thrombocytopenia, and autoimmune haemolytic anaemia. They are usually negative for anti-RNP and anti-Sm antibodies (7). The findings in the patient described in this study were consistent in this regard such that the prominent abnormalities were serological, and the symptoms were unremarkable except for a mild involvement with the central nervous system.
Interestingly, the features in the patient with KS in this study overlapped with IgG4-RD. An overlap of SLE and IgG4-RD is rare; only four patients with such a presentation have been reported in the literature (9-12). The clinical characteristics of all those patients including that in our case are summarized in Table 2. The onset of IgG4-RD and SLE were concurrent in three cases, SLE preceded IgG4-RD in one of the cases and IgG4-RD preceded SLE in another case. The diagnosis of the two diseases was made concurrently in all the cases at 58 years of age which is the susceptible age for IgG4-RD. Similar to SLE, a female predilection was observed. As there were no detectable similarities in the clinical features in the five patients, the complications observed are likely to be occult. However, there are several reports on overlapping cases of IgG4-RD and Sjögren syndrome, ANCA-associated vasculitis and relapsing polychondritis, suggesting that IgG4-RD can share some heterogenous autoimmune conditions (13-16). Moreover, GWAS conducted for IgG4-RD recently revealed that this condition shares several disease susceptibility genes with SLE, such as human leukocyte antigen (HLA)-DRB1 (p = 1.1×10-11 for IgG4-RD; odds ratio of 2.4 for SLE) and FCGR2B (p = 2.0×10-8 for IgG4-RD, odds ratio of 2.3-2.45 for SLE) (23, 24, 27-29), thereby suggesting the common genetic factors associated with the development of the two diseases, which may be independent of KS because these two genes are not located on X chromosome. Although it is difficult to prove the effect of X chromosome on IgG4-RD development, one of its explanations on the pathogenesis is an overexpression of CD40 ligand (CD40L) which has been reported on the T cells of KS patients (22). The interaction between CD40 and CD40L is essential to elicit a T cell-dependent B cell response as well as immunoglobulin class switching (30). In SLE, CD40-CD40L interaction is well known to be associated with its pathogenesis and it is regarded as a potential therapeutic target (31). Although there has been no report focusing on CD40L expression in IgG4-RD, its overexpression may contribute to a class-switch to IgG4 and plasma cell differentiation.
Table 2.
Overlapping Cases of IgG4-related Disease and Systemic Lupus Erythematous.
| Case | Case 1 [9] | Case 2 [10] | Case 3 [11] | Case 4 [12] | Present case |
|---|---|---|---|---|---|
| Sex | F | F | F | F | M (46XY/47XXY mosaic) |
| Comorbidities | none | none | none | none | Klinefelter syndrome |
| Manifestations | fever, skin rash, proteinuria abdominal pain | fever, proteinuria | weight loss, hair loss, arthralgia, lymphadenopathy | abdominal pain, diarrhea, vomiting, proteinuria | lower leg ulcer, ataxia |
| Age (onset) | 37 | 58 | 60 | 71 | 46 |
| Age (diagnosis) | 37 | 58 | 63 | 71 | 54 |
| ANA, Pattern | 2,560 folds, N/A | 640 folds, diffuse and nuclear | 80 folds, homogeneous | 320 folds, homogeneous | 1,280 folds, homogeneous and speckled |
| Anti-dsDNA antibody, U/mL | >300 | 18 | 1:40 (immunofluorescence) | negative | 24 |
| Anti-Sm antibody, U/mL | 160.4 | N/A | negative | negative | negative |
| CH50, U/mL | <10 | <10 | N/A | N/A | <10 |
| C3, mg/dL | 50 | N/A | WNL | 100 | 18 |
| C4, mg/dL | 4 | N/A | WNL | 11 | 2 |
| IgG, mg/dL | 1,920 | 2,719 | N/A | 2,420 | 4,727 |
| IgG4, mg/dL | 224 | 1,240 | 452 | 37 | 1,826 |
| IgG4/IgG, % | 11.7 | 45.6 | N/A | 1.5 | 38.6 |
| IgG4-RD RI | 6 | 9 | 6 | 3 | 18 |
| Biopsy | kidney | kidney | lymph node | kidney | skin, kidney, lymph node |
| Organ involvement | skin, kidney, pancreas | kidney, lymph node | joint, hair, lymph node | kidney | central nerve system, skin, kidney, lymph node, bile duct |
| Treatment | methylprednisolone pulse 1,000 mg/day (3days)→ PSL 40 mg/day | PSL 40 mg/day, mycophenolate mofetil, belimumab | PSL 10mg /day, hydroxychloroquine 200 mg/day | methylprednisolone pulse 1,000 mg/day 3days→PSL 60 mg/day, mycophenolate mofetil |
PSL 60 mg/day, intravenous cyclophosphamide 750 mg/m2 |
| Prognosis | resolved | resolved | resolved | resolved | resolved |
ANA: anti-nuclear antibody, IgG4-RD: IgG4-related disease, IgG4-RD RI: IgG4-related disease responder index, LN: lupus nephritis, N/A: not available, PSL: prednisolone, SLE: systemic lupus erythematosus, TIN: tubulointerstitial nephritis, WNL: within normal limits
The diagnosis of both SLE and IgG4-RD is sometimes difficult especially in atypical cases, such as those that demonstrate some disease overlap. This is because both conditions affect people of diverse subpopulations and involve multiple organ systems. Thorough the examination of the patient in this study helped to infer that either diagnosis could not explain all his manifestations. Hypocomplementemia in our patient could be either to IgG4-RD or SLE determined. Although the emergence of immune complex in the circulation with complement consumption is typical feature of SLE (32), those phenomena can be also observed in IgG4-RD (33). Nevertheless, a typical case with IgG4-RD usually does not show such a high titer of anti-nuclear antibody (ANA), anti-dsDNA antibody, and positive direct coombs test. We therefore the final diagnosis of this patient was IgG4-RD and KS with lupus-like serological and neurological features, but careful observation with occasional pathological examinations will be needed to make a precise diagnosis in such atypical cases.
We herein described a rare overlapping case of IgG4-RD and KS with lupus-like serological and neurological features. The findings presented in this study are interesting and suggestive of a common pathology between the diseases. Further studies are essential to unravel the pathogenic mechanisms underlying these diseases.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Groth KA, Skakkebæk A, Høst C, Gravholt CH, Bojesen A. Clinical review: Klinefelter syndrome-a clinical update. J Clin Endocrinol Metab 98: 20-30, 2013. [DOI] [PubMed] [Google Scholar]
- 2. Nielsen J, Wohlert M. Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Hum Genet 87: 81-83, 1991. [DOI] [PubMed] [Google Scholar]
- 3. Scofield RH, Bruner GR, Namjou B, et al. . Klinefelter's syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum 58: 2511-2517, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seminog OO, Seminog AB, Yeates D, Goldacre MJ. Associations between Klinefelter's syndrome and autoimmune diseases: English national record linkage studies. Autoimmunity 48: 125-128, 2015. [DOI] [PubMed] [Google Scholar]
- 5. Tsokos GC. Systemic lupus erythematosus. N Engl J Med 365: 2110-2121, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab 91: 1254-1260, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Dillon S, Aggarwal R, Harding JW, et al. . Klinefelter's syndrome (47,XXY) among men with systemic lupus erythematosus. Acta Paediatr 100: 819-823, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet 385: 1460-1471, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi S, Yoshida M, Kitahara T, et al. . Autoimmune pancreatitis as the initial presentation of systemic lupus erythematosus. Lupus 16: 133-136, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Yamamoto M, Aochi S, Suzuki C, et al. . A case with good response to belimumab for lupus nephritis complicated by IgG4-related disease. Lupus 28: 786-789, 2019. [DOI] [PubMed] [Google Scholar]
- 11. Naramala S, Biswas S, Adapa S, Gayam V, Konala VM. An overlapping case of IgG4-related disease and systemic lupus erythematosus. J Investig Med High Impact Case Rep. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zaarour M, Weerasinghe C, Eter A, El-Sayegh S, El-Charabaty E. An overlapping case of lupus nephritis and IgG4-related kidney disease. J Clin Med Res 7: 575-581, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakashima Y, Nakamura H, Horai Y, et al. . Comorbid case of IgG4-related disease and primary Sjögren's syndrome. Mod Rheumatol 25: 462-467, 2015. [DOI] [PubMed] [Google Scholar]
- 14. Huang X, Lu B, Li M, Fan Y, Zhang L. IgG4-related retroperitoneal fibrosis overlapping with primary biliary cirrhosis and primary Sjögren's syndrome: a case report. Medicine (Baltimore) 97: e11303, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Danlos FX, Rossi GM, Blockmans D, et al. . Antineutrophil cytoplasmic antibody-associated vasculitides and IgG4-related disease: a new overlap syndrome. Autoimmun Rev 16: 1036-1043, 2017. [DOI] [PubMed] [Google Scholar]
- 16. Nagayama Y, Takayasu M, Wakabayashi A, et al. . New onset of immunoglobulin G4-related disease in a patient with relapsing polychondritis. Mod Rheumatol 27: 898-900, 2017. [DOI] [PubMed] [Google Scholar]
- 17. Schmitz-Hübsch T, du Montcel ST, Baliko L, et al. . Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66: 1717-1720, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Umehara H, Okazaki K, Masaki Y, et al. . Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol 22: 21-30, 2012. [DOI] [PubMed] [Google Scholar]
- 19. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725, 1997. [DOI] [PubMed] [Google Scholar]
- 20. Aringer M, Costenbader K, Daikh D, et al. . 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 78: 1151-1159, 2019. [DOI] [PubMed] [Google Scholar]
- 21. Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434: 400-404, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Sarmiento L, Svensson J, Barchetta I, Giwercman A, Cilio CM. Copy number of the X-linked genes TLR7 and CD40L influences innate and adaptive immune responses. Scand J Immunol 90: e12776, 2019. [DOI] [PubMed] [Google Scholar]
- 23. Moser KL, Kelly JA, Lessard CJ, Harley JB. Recent insights into the genetic basis of systemic lupus erythematosus. Genes Immun 10: 373-379, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graham RR, Ortmann WA, Langefeld CD, et al. . Visualizing human leukocyte antigen class II risk haplotypes in human systemic lupus erythematosus. Am J Hum Genet 71: 543-553, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Souyris M, Cenac C, Azar P, et al. . TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 26. Ishiguro N, Moriyama M, Furusho K, et al. . Activated M2 macrophages contribute to the pathogenesis of IgG4-related disease via toll-like receptor 7/interleukin-33 signaling. Arthritis Rheumatol 72: 166-178, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Terao C, Ota M, Iwasaki T, et al. . IgG4-related disease in the Japanese population: a genome-wide association study. Lancet Rheumatol 1: e14-e22, 2019. [DOI] [PubMed] [Google Scholar]
- 28. Kyogoku C, Dijstelbloem HM, Tsuchiya N, et al. . Fcgamma receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus: contribution of FCGR2B to genetic susceptibility. Arthritis Rheum 46: 1242-1254, 2002. [DOI] [PubMed] [Google Scholar]
- 29. Chu ZT, Tsuchiya N, Kyogoku C, et al. . Association of Fcgamma receptor IIb polymorphism with susceptibility to systemic lupus erythematosus in Chinese: a common susceptibility gene in the Asian populations. Tissue Antigens 63: 21-27, 2004. [DOI] [PubMed] [Google Scholar]
- 30. Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci 58: 4-43, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murphy G, Isenberg DA. New therapies for systemic lupus erythematosus - past imperfect, future tense. Nat Rev Rheumatol 15: 403-412, 2019. [DOI] [PubMed] [Google Scholar]
- 32. Fava A, Petri M; Systemic lupus erythematosus. Diagnosis and clinical management. J Autoimmun 96: 1-13, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muraki T, Hamano H, Ochi Y, et al. . Autoimmune pancreatitis and complement activation system. Pancreas 32: 16-21, 2006. [DOI] [PubMed] [Google Scholar]