Figure 1.
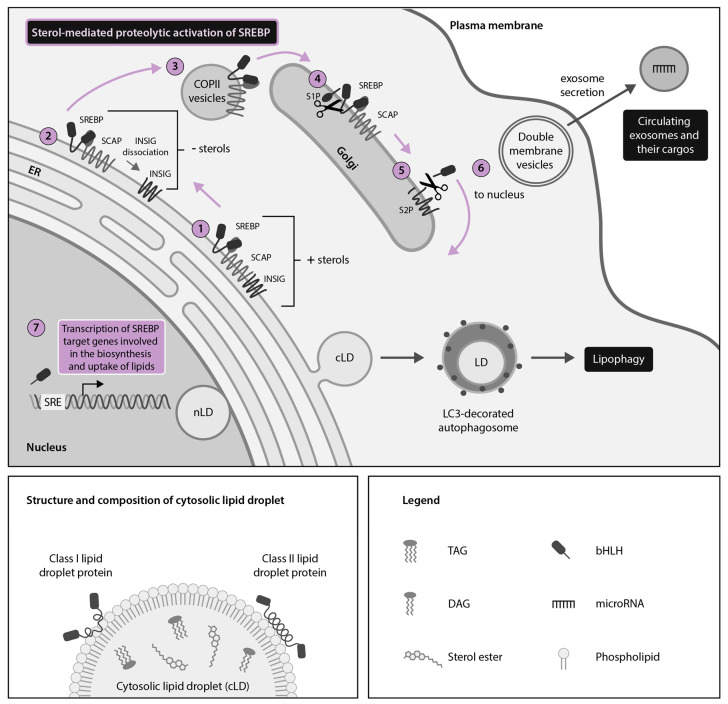
The sterol regulatory element-binding protein (SREBP) pathway is a master regulator of cellular lipid droplet (LD) metabolism. LDs are spherical organelles composed of a phospholipid monolayer surrounding a core of neutral lipids. LDs have been described in both the cytoplasm (cLDs) and nucleus (nLDs) of homeostatic cells, with cLDs being better described. cLDs serve as docking platforms for Class I and Class II host LD proteins. LD metabolism is controlled by endoplasmic reticulum (ER)- and nuclear membrane-bound proteins called SREBPs, which control expression of key target genes involved in the biosynthesis and uptake of cholesterol and other lipids. When sterol levels are high, SREBPs are retained in an inactive state in the ER by SREBP-cleavage activating protein (SCAP) and insulin-induced gene (INSIG) (1). When sterol levels are low, INSIG dissociates from SCAP at the ER (2) and coat protein II (COPII)-coated vesicles transport SREBP-SCAP complexes to the Golgi apparatus (3), where the active N-terminal portion of SREBPs is cleaved by site-1 protease (S1P) (4) and site-2 protease (S2P) (5). The N-terminal portion of SREBPs then translocates to the nucleus (6) to act as a transcription factor coordinating expression of many lipid-metabolism related genes (7) that can subsequently impact LD biogenesis. SREBP regulation of LD metabolism can be further fine-tuned by exosome-packaged host microRNAs (miR) such as miR-29 (inhibits SCAP and SREBP-1 expression), miR-33 (inhibits SREBP-1 expression) and miR-24 (inhibits INSIG expression). SREBP-regulated LD homeostasis also intersects with other cellular pathways such as lipophagy, which catabolizes LDs in microtubule-associated protein light chain 3 (LC3)-decorated autophagosomes to liberate their component lipids.