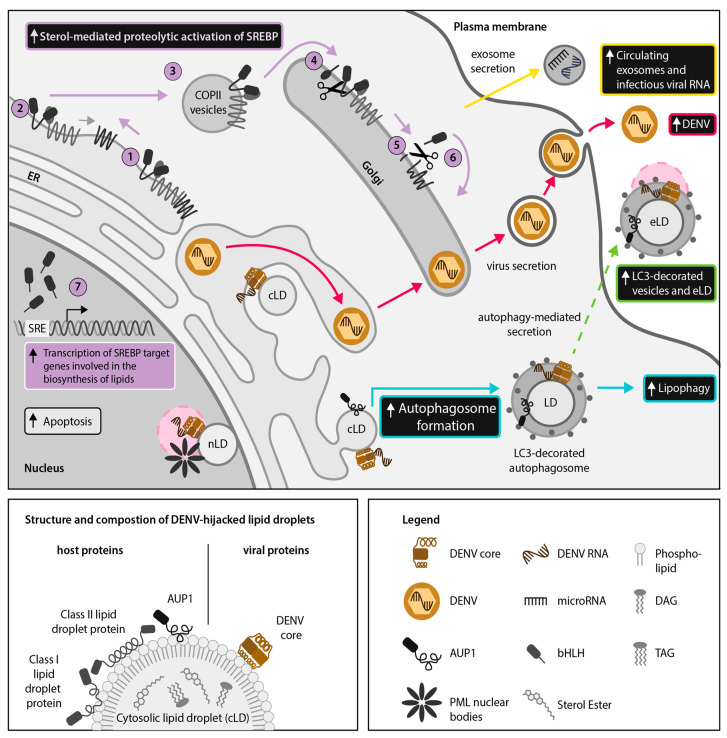
Figure 3.
Dengue virus (DENV) dysregulates host lipid metabolism. DENV perturbs host lipid homeostasis by enhancing sterol regulatory element-binding protein (SREBP) activation (purple arrows: (1–7)), leading to increased lipid droplet (LD) formation. DENV stimulates LD degradation via lipophagy by enhancing (ancient ubiquitous protein 1) AUP1 translocation from cytoplasmic LDs (cLDs) to microtubule-associated protein light chain 3 (LC3)-decorated autophagosomes (cyan arrows). Enhanced LD catabolism provides free fatty acids to serve as an energy source for production of infectious virions (red arrows). A recent report has shown that infectious viral RNA and extracellular LDs (eLDs) are contained within LC3-decorated extracellular vesicles in DENV-infected cells, suggesting that eLDs could be used as anchors for viral proteins. We hypothesize that the cLD-associated DENV core protein may also co-localize with eLDs in order to facilitate extracellular dissemination of viral RNA (green arrows). In addition, we propose that the DENV core protein may utilize nLDs as a scaffold for interacting with promyelocytic leukemia (PML) nuclear bodies (pink circle), thereby promoting host cell apoptosis and suppressing host interferon responses. Hypothetical pathways are highlighted with dotted lines.