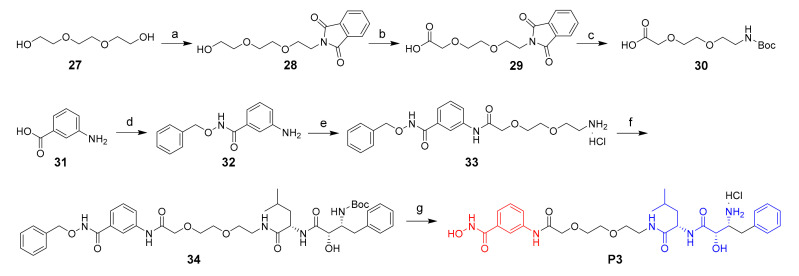
Scheme 3.
Reagents and conditions: (a) isoindoline-1,3-dione, PPh3, DIAD, THF, 57%; (b) PhI(AcO)2, TEMPO, CN/H2O, 70 °C; (c) 1) N2H4.H2O, EtOH, 80 °C; 2) (Boc)2O, NaOH, THF; yield: 41% for two steps; (d) O-benzylhydroxylamine hydrochloride, HATU, DIPEA, DMF, 71%; (e) 1) 30, HATU, DIPEA, DMF; 2) HCl/dioxane; (f) 14, HATU, DIPEA, DMF, 50%; (g) 1) 10% Pd/C, H2, MeOH; 2) HCl/dioxane; yield: 8% for two steps.