Figure 2.
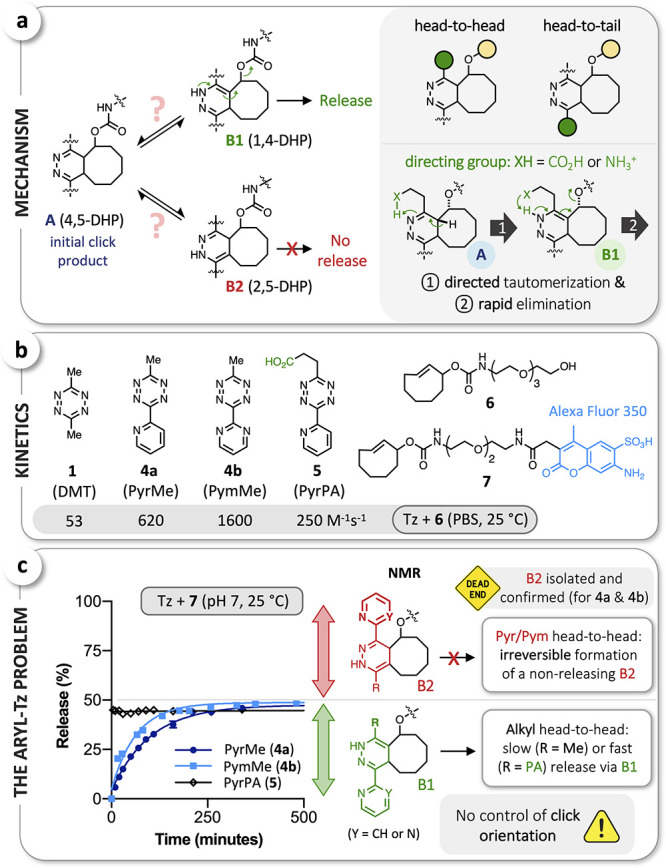
(a) The critical role of postclick tautomerization (left; DHP = dihydropyridazine); directed and accelerated formation of B1 as well as enhanced 1,4-elimination with directing groups (CO2H, NH3+) in head-to-head position (right). (b) Stopped-flow measurements verified the significantly higher click reactivity of aryl-Tz 4a, 4b, and 5 with the water-soluble rTCO-derivative 6. (c) Investigation of the reactions of PyrMe (4a), PymMe (4b), and PyrPA (5) with rTCO-Alexa Fluor 350 (7) by LCMS revealed the irreversible formation of B2 tautomers with Pyr/Pym in head-to-head position, as confirmed by NMR for the reactions of 4a and 4b with rTCO-glycine49 (see Supporting Information).
