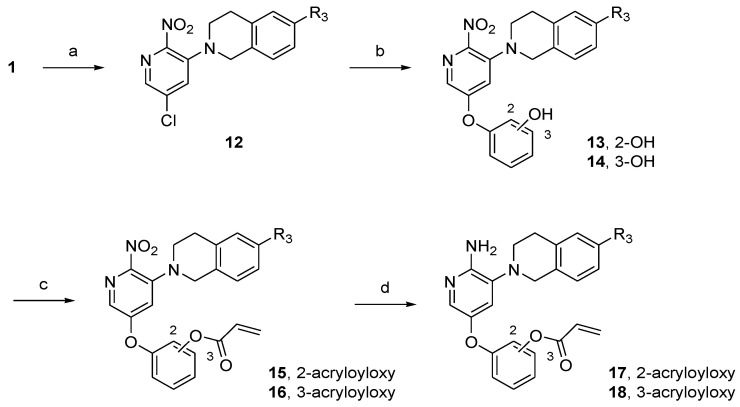
Scheme 3.
Synthesis of tetrahydroisoquinoline-linked aminopyridine derivatives. Reagents and conditions: (a) 11a–f or 1,2,3,4-tetrahydroisoquinoline, K2CO3, toluene, 60 °C, 10 h, 34–57%; (b) resorcinol or catechol, K2CO3, dimethyl sulfoxide (DMSO), 18-crown-6, 100 °C, 3 h, 25–74%; (c) acryloyl chloride, TEA, DCM, rt, 1 h, 30–99%; (d) SnCl2∙2H2O, THF/EtOH, rt, 1 h, 4–67%.