Figure 1. Structure and mechanism of domain swapping in FoxP proteins.
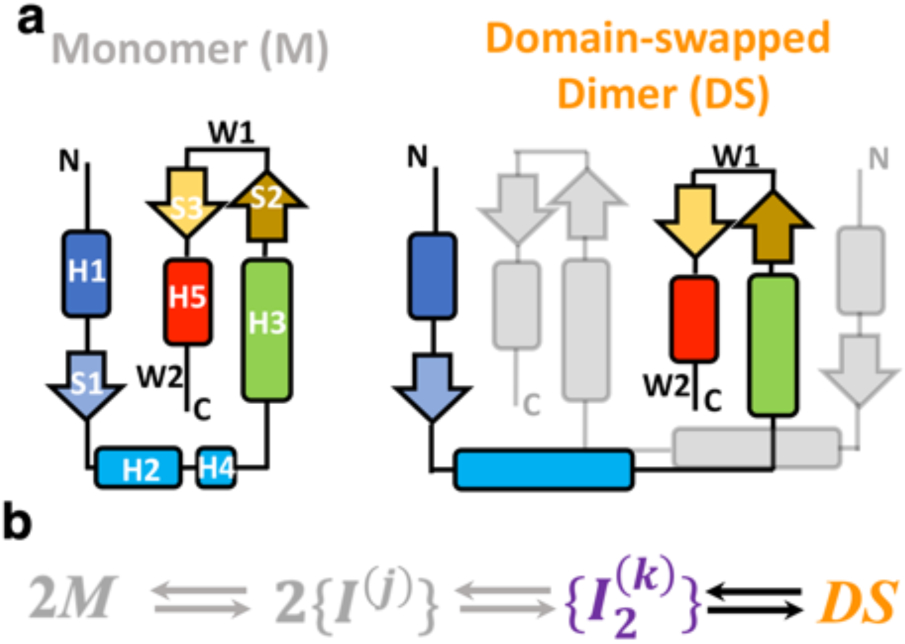
a) Topology of the monomer and domain-swapped dimer (DS) of FoxP proteins. Secondary structure elements are represented with different colors in order to clarify the topological changes between monomer and dimer. Helices H2 and H4 in the dimer are fused in the dimer. b) Kinetic scheme highlighting the inferred association/dissociation steps. According to previous studies, the dimerization of FoxP proteins from native monomers (M) proceeds toward the formation of monomeric intermediates ({I(j)) that must adopt different conformations, constituting observed ensembles of states. In this scenario, the adoption of the DS could be promoted by the formation of dimeric and heterogeneous intermediate ensembles ({I2(k)}).
