 |
10. Red solid (73%), mp 85 °C. 1H NMR (300 MHz, CDCl3) δ 7.71–7.60 (m, 2H, arom), 7.35 (dd, J = 7.6, 3.8 Hz, 4H, arom), 7.28 (s, 1H, arom), 7.03 (d, J = 8.3 Hz, 2H, arom). 13C NMR (75 MHz, CDCl3) δ 134.6, 131.7, 131.3, 130.7, 129.4, 129.0, 128.4, 127.1, 126.2, 123.1. |
 |
11. Red solid (79%), mp 90 °C. 1H NMR (300 MHz, CDCl3) δ 7.69 (d, J = 8.2 Hz, 2H, arom), 7.42 (d, J = 8.3 Hz, 2H, arom), 7.26 (d, J = 8.2 Hz, 2H, arom), 7.13 (d, J = 8.3 Hz, 2H, arom), 2.42 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3) δ 162.3, 151.2, 150.9, 142.5, 134.7, 131.4, 131.0, 129.7, 128.4, 123.2, 21.5. Crystals, suitable for X-ray analysis, were obtained by the slow evaporation of saturated hexane/EtOAc (5/1) solution. |
 |
12. Red solid (72%), mp 96 °C. 1H NMR (300 MHz, CDCl3) δ 7.78 (d, J = 9.0 Hz, 2H, arom), 7.42 (d, J = 8.4 Hz, 2H, arom), 7.13 (d, J = 8.4 Hz, 2H, arom), 6.95 (d, J = 9.0 Hz, 2H, arom), 3.88 (s, 3H, OCH3). 13C NMR (75 MHz, CDCl3) δ 162.7, 162.3, 151.1, 147.2, 134.6, 131.4, 131.2, 128.4, 125.3, 114.2, 55.6. |
 |
13. Red solid (68%), mp 77 °C. 1H NMR (300 MHz, CDCl3) δ 7.81 (dd, J = 8.6, 5.4 Hz, 2H), 7.43 (d, J = 8.3 Hz, 2H), 7.14 (t, J = 8.8 Hz, 4H). 13C NMR (75 MHz, CDCl3) δ 167.6, 166.4, 151.1, 149.3, 134.8, 131.4, 130.8, 129.6, 128.5, 125.4, 116.2, 115.9. Crystals, suitable for X-ray analysis, were obtained by the slow evaporation of saturated hexane/EtOAc (5/1) solution. |
 |
14. Red solid (67%), mp 94 °C. 1H NMR (300 MHz, CDCl3) δ 7.73 (d, J = 8.6 Hz, 1H), 7.43 (d, J = 8.4 Hz, 2H), 7.12 (d, J = 8.4 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 162.3, 151.1, 137.7, 136.5, 134.9, 131.4, 130.6, 129.3, 128.5, 124.4. Crystals, suitable for X-ray analysis, were obtained by the slow evaporation of saturated hexane/EtOAc (5/1) solution. |
 |
15. Red solid (70%), mp 105 °C. 1H NMR (300 MHz, CDCl3) δ 7.69–7.56 (m, 4H, arom), 7.49–7.39 (m, 2H, arom), 7.12 (d, J = 8.4 Hz, 2H, arom). 13C NMR (75 MHz, CDCl3) δ 151.4, 134.9, 132.3, 131.4, 130.6, 129.8, 128.5, 127.4, 126.3, 124.6. Crystals, suitable for X-ray analysis, were obtained by the slow evaporation of saturated hexane/EtOAc (5/1) solution. |
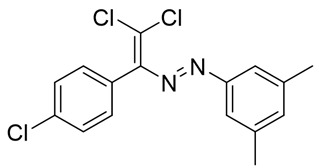 |
16. Red solid (82%), mp 145 °C. 1H NMR (300 MHz, CDCl3) δ 7.44 (d, J = 7.7 Hz, 4H, arom), 7.15 (d, J = 7.7 Hz, 3H, arom), 2.40 (s, 6H, CH3). 13C NMR (75 MHz, CDCl3) δ 157.7, 148.3, 146.7, 134.2, 130.2, 129.0, 126.8, 126.5, 123.9, 116.5, 16.6. |
 |
17. Red solid (66%), mp 115 °C. 1H NMR (300 MHz, CDCl3) δ 7.89 (s, 1H, arom), 7.68–7.61 (m, 1H, arom), 7.54 (d, J = 8.6 Hz, 1H, arom), 7.44 (d, J = 8.3 Hz, 2H, arom), 7.11 (d, J = 8.3 Hz, 2H, arom). 13C NMR (75 MHz, CDCl3) δ 151.5, 151.4, 135.7, 135.0, 133.5, 131.3, 130.8, 130.4, 129.8, 128.6, 124.5, 122.7. Crystals, suitable for X-ray analysis, were obtained by the slow evaporation of saturated hexane/EtOAc (5/1) solution. |
 |
18. Red solid (71%), mp 121 °C. 1H NMR (300 MHz, CDCl3) δ 7.57 (d, J = 8.7 Hz, 1H, arom), 7.46 (d, J = 2.0 Hz, 1H, arom), 7.37 (d, J = 8.4 Hz, 2H, arom), 7.24 (d, J = 2.3 Hz, 1H, arom), 7.12 (d, J = 8.4 Hz, 2H, arom). |

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.