Table 1.
N-arylamine oxetanes obtained via Buchwald–Hartwig Reaction.
| Entry | Halide/Triflate | Product | Yield (%) a |
|
|---|---|---|---|---|
| 1 |
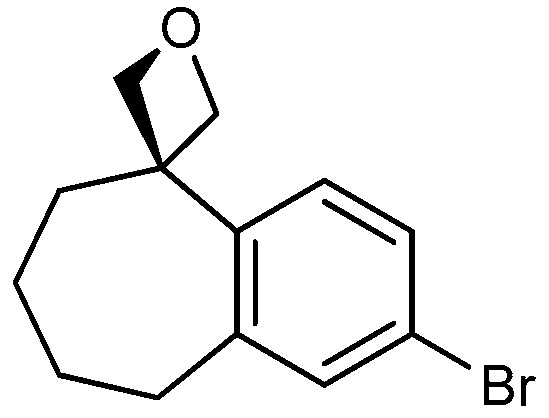
|
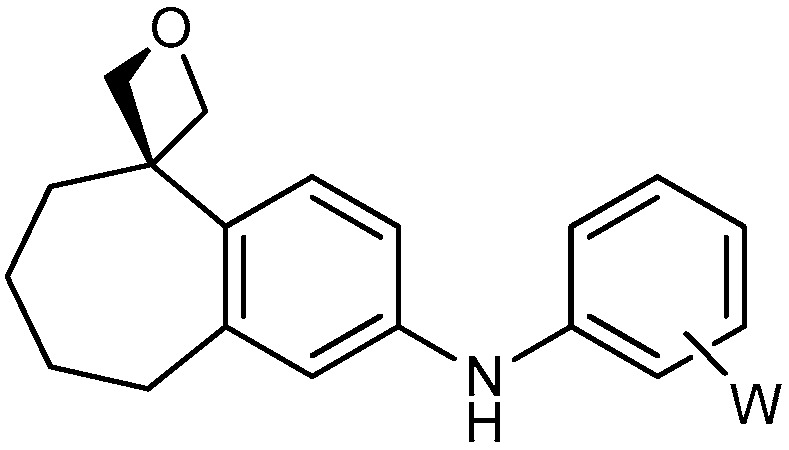
|
16a: W = H 16b: W = 2,4-di-F 16c: W = 2-CF3 16d: W = 4-OCH3 |
86 73 77 86 |
| 2 |
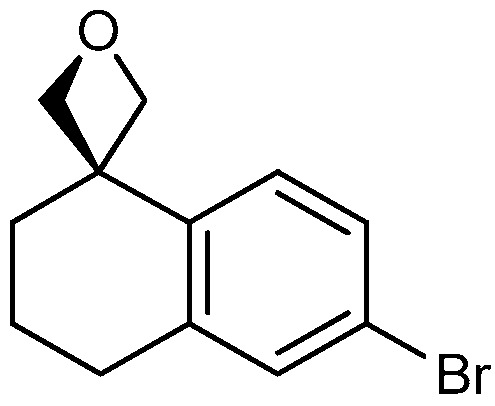
|
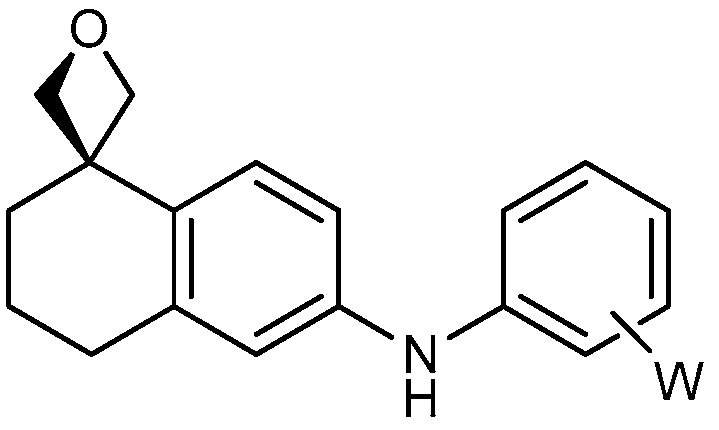
|
17a: W = H 17b: W = 2,4-di-F 17c: W = 2-CF3 17d: W = 2-OCH3 |
92 51 82 82 |
| 3 |
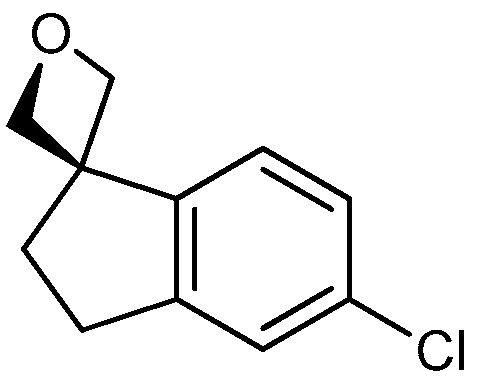
|
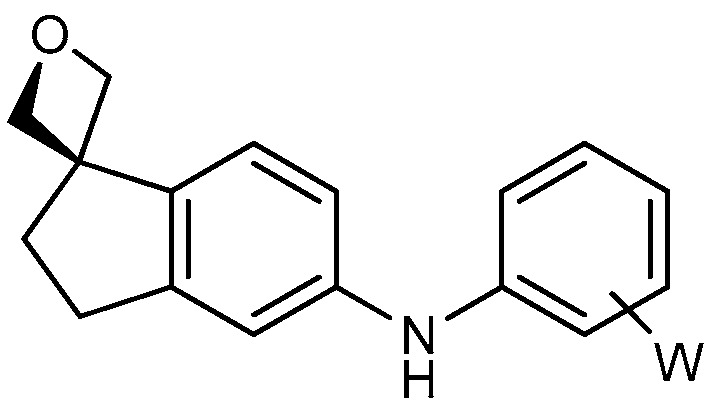
|
18a: W = H 18b: W = 2,4-di-F 18c: W = 2-CF3 18d: W = 2-OCH3 |
71 76 95 80 |
| 4 |
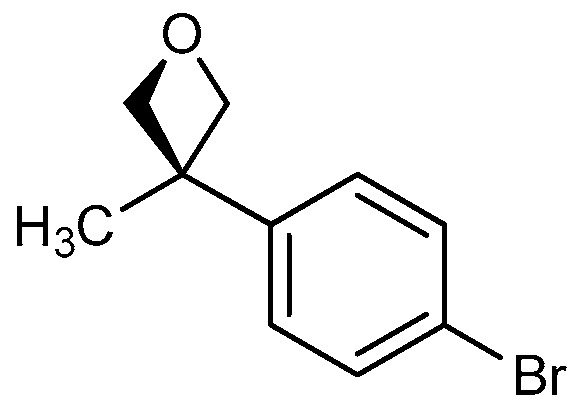
|
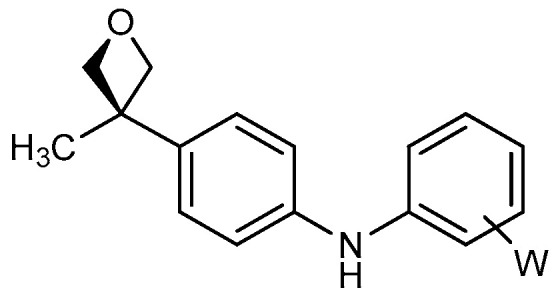
|
19a: W = H 19b: W = 2,4-di-F 19c: W = 2-CF3 19d: W = 2-OCH3 |
83 71 88 81 |
| 5 b |
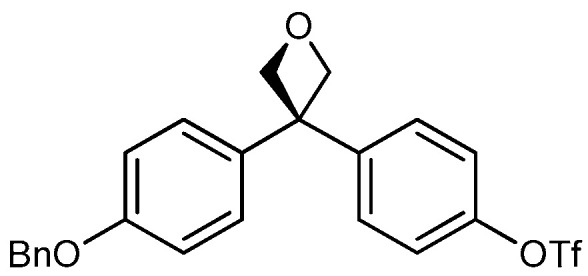
|
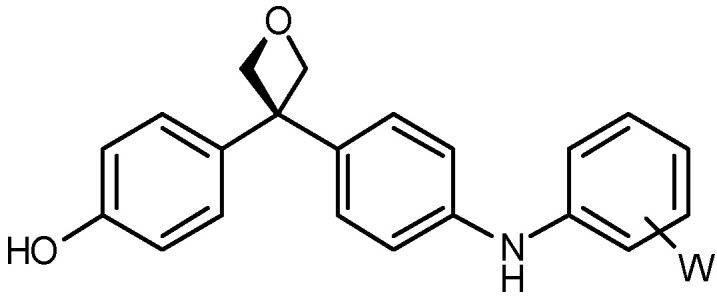
|
20a: W = H 20b: W = 2,4-di-F 20c: W = 4-CF3 20d: W = 4-OCH3 20e: W = 4-CN |
85 66 71 54 70 |
a Isolated yields after purification by column chromatography. b Products obtained after deprotection of benzyl ether group.
