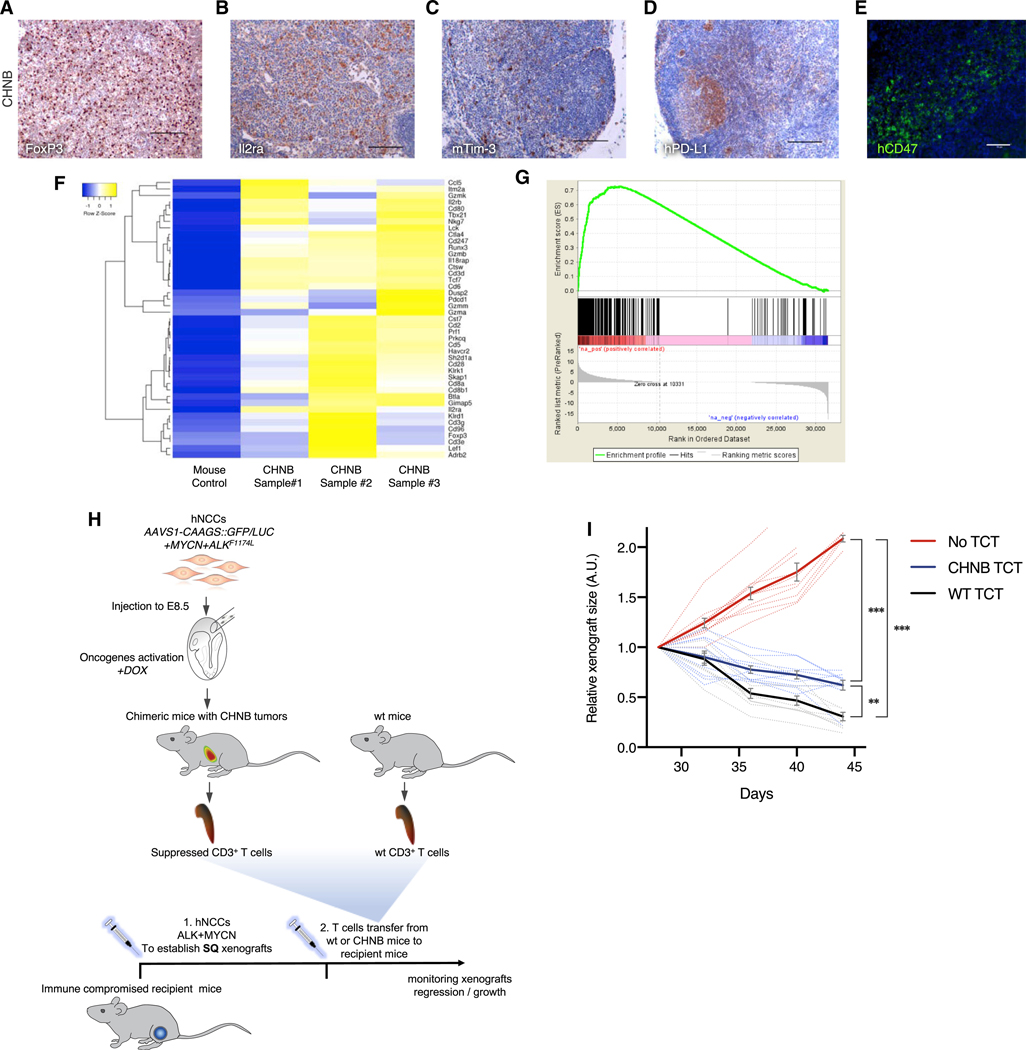
Figure 7. Immune-Evasion and Immune-Suppressed T Cells in NB Tumors from Mouse-Human Chimeras.
(A and B) Human NB tumor of chimeric mice shows immune tolerance marked by infiltration of Tregs (A, stained for FoxP3) and activated T cells (B, Il2ra).
(C–E) T cell exhaustion was observed in NB chimeric mice, indicated by the expression of immune checkpoint signals, such as the mouse-specific Tim-3 on the hosts T cells (C) and human-specific PD-L1 (D, the ligand for PD1 expresses on T cells; IHC scale bars indicate 100 mm) and human-specific CD47 on human tumor cell (E, activating “don’t eat me” signals on macrophages; IF scale bar indicates 50 μm).
(F) RNA-seq analysis for the murine genes expressed in CHNB tumor (represent the tumor microenvironment) show a gene expression signature with enrichment for genes associated with T cell immune activation and exhaustion.
(G) Similarly, the enrichment plot of gene set enrichment analysis (GSEA) of the tumor microenvironment-associated genes (mouse reads) from CHNBs show an expression signature significantly associated with cancer-linked inflammation response.
(H) Schematic representation of the experiment. T cells from chimeric mice bearing CHNBs and CD3+ T cells from WT mice were transferred to recipient immunocompromised mice, which were challenged with subcutaneous xenograft injection of hNCCs overexpressing MYCN and ALK (see Figure 1G). Xenografts’ growth were monitored for their size following the T cells transfers (TCT) as measurement for T cell acclivity.
(I) hNCCs xenografts in immune-compromised mice with no TCT kept growing rapidly (red). Xenograft tumors in mice injected with CD3+ cells from CHNB-bearing mice grew significantly slower than in mice transferred with WT T cells (n = 10 per group; solid lines represent means; error bars represent SD, ** p value < 0.001; *** p value < 0.0001, repeated-measures ANOVA).