Fig. 1.
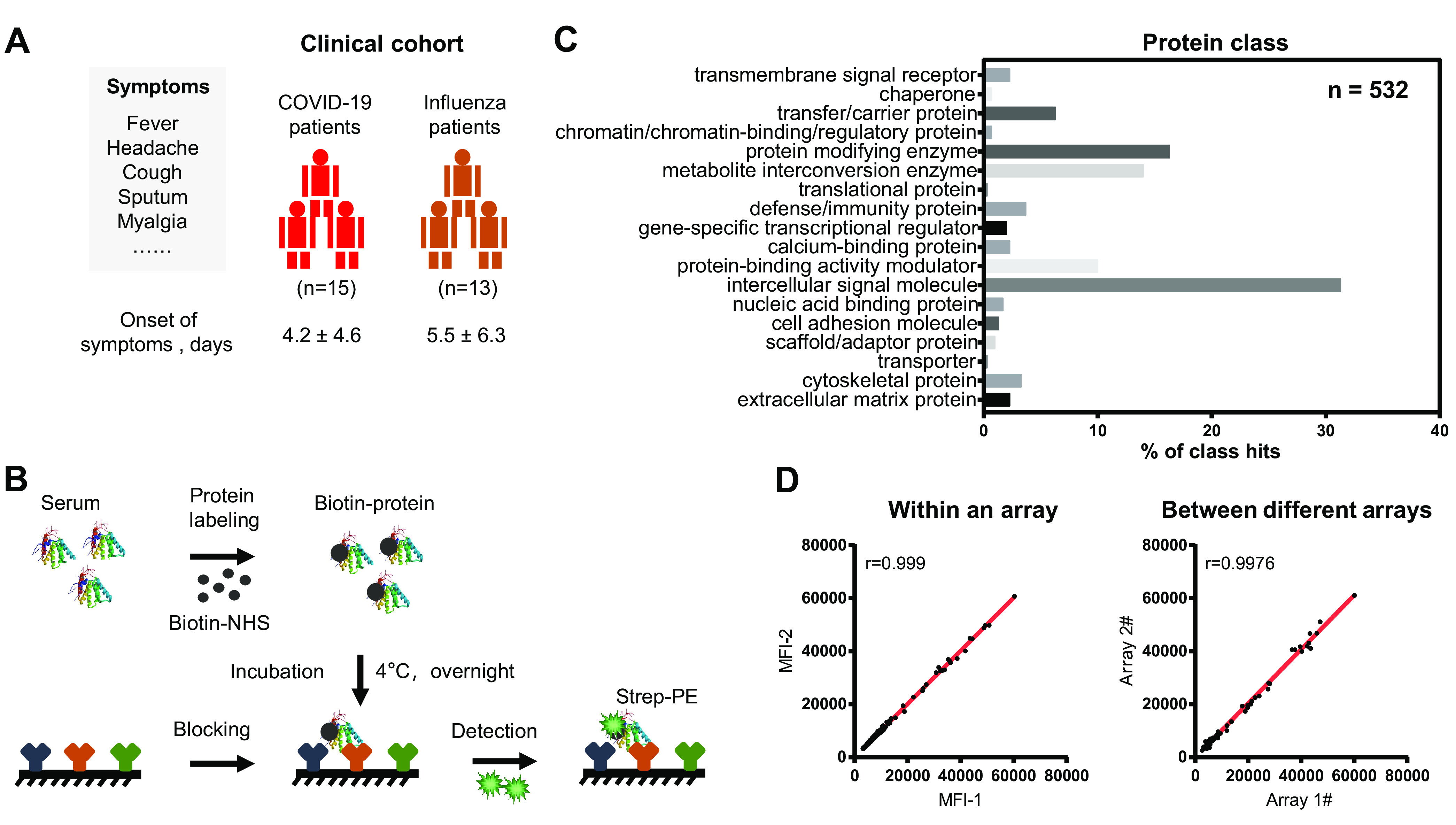
Schematic illustration of serum screening of early-stage COVID-19 patients. A, Characteristics of the clinical cohort used in this study. All enrolled COVID-19 patients displayed with the symptoms (i.e. fever, headache, cough sputum, myalgia) were diagnosed in accordance to the Diagnosis and Management Plan of Pneumonia with Novel Coronavirus Infection (trial version 7). The detail clinical information of each COVID-19 patient is shown in the supplemental Table S1. B, Workflow for serum screening using the antibody microarray. The proteins in serum was labeled with biotin and incubated with the blocked antibody microarray overnight at 4 °C. The biotin-proteins captured on microarray can be detected with streptavidin-Phycoerythrin (strep-PE) at the wavelength of 532 nm. C, Classes of proteins on the antibody microarray. GO analysis (protein class) was performed using the PANTHER database (http://pantherdb.org/). D, Reproducibility of the antibody microarray for the detection of serum proteins. The correlation (r) within an array and different arrays were calculated using the microarray data within an experiment and different experiments, respectively.
