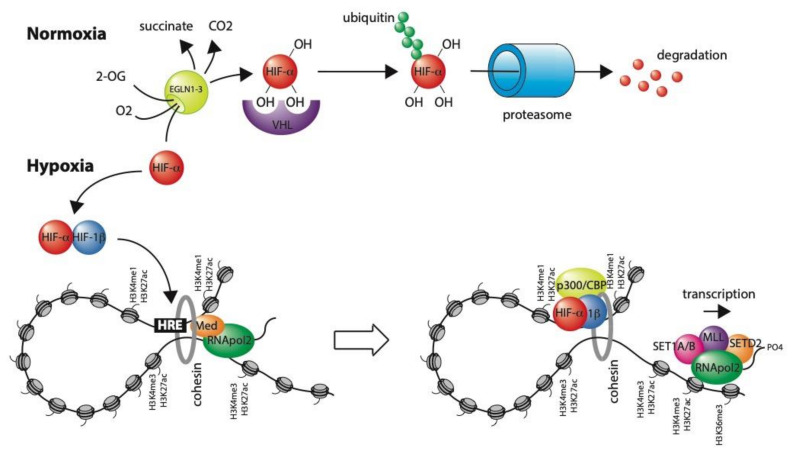
Figure 1.
Overview of the HIF transcription factor in normoxia and hypoxia. In normal oxygen conditions (normoxia), the oxygen-dependent prolyl hydroxylases (EGLN1-3) hydroxylate HIF-α. This hydroxylation allows the von Hippel-Lindau E3 ubiquitin ligase to bind HIF-α and to covalently add ubiquitin moieties that target it for proteasomal degradation. In low levels of oxygen (hypoxia), the activity of the EGLN enzymes is reduced and HIF-α is stabilized. It then forms a heterodimer with HIF-1β and translocates to the nucleus, where it binds to hypoxia response elements (HREs) to release promoter-paused RNApol2 and enhance gene transcription. Often, these HREs are in distant enhancer regions, which contact their target promoters through cohesin-mediated chromatin looping. In normoxia, before HIF is stabilized, HIF-binding sites and target promoters are generally accessible and display histone modifications associated with active enhancers and promoters. However, both HIF and activated RNApol2 recruit additional essential epigenetic modifying activities that further modify the chromatin as a result of HIF-mediated transactivation.