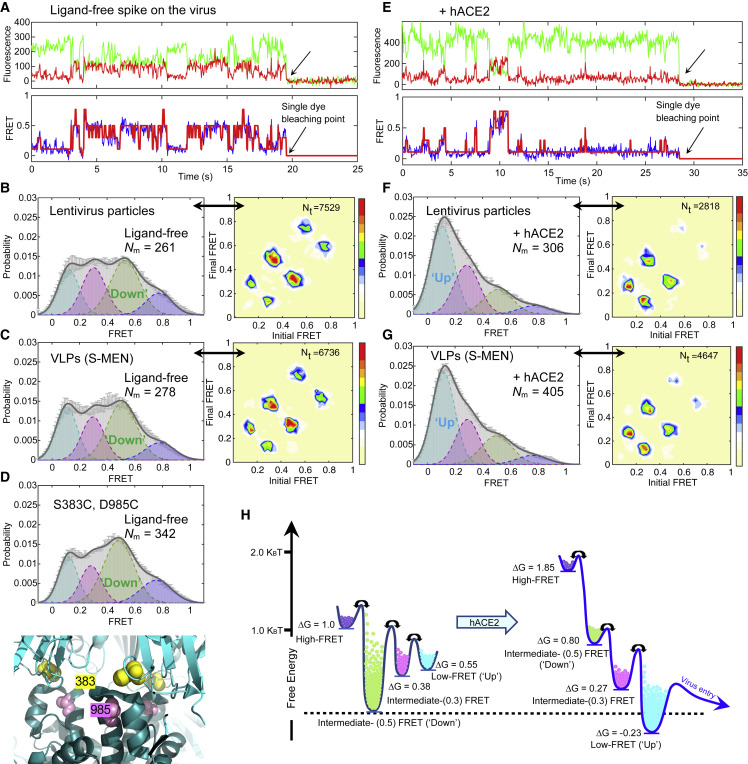
Figure 2.
SARS-CoV-2 Spike Protein Is Dynamic, and hACE2 Shifts Conformational Landscape from the Ground State to the Receptor-Bound State through One Necessary Intermediate
(A–D) The ligand-free S on virus particles primarily resides in “RBD-down” conformation (ground state).
(A) Example fluorescence trace (Cy3B, green; LD650, red) and resulting quantified FRET traces (FRET efficiency, blue; hidden Markov model initialization, red) of a dually labeled ligand-free spike protein on the surface of HIV-1 lentivirus particle. Arrows point to the single-step photobleaching steps of dyes at the single-molecule level and define the baseline.
(B and C) FRET histograms (left) and TDPs (right) of ligand-free spikes on lentivirus particles (B) and S-MEN viral-like particles (C). Also shown is the number (Nm) of individual dynamic molecules/traces compiled into a conformation-population FRET histogram (gray lines) and fitted into a 4-state Gaussian distribution (solid black) centered at 0.1-FRET (dashed cyan), 0.3-FRET (dashed red), 0.5-FRET (dashed green), and 0.8-FRET (dashed magenta). TDPs, displayed as initial FRET versus final FRET with relative frequencies, trace the locations of state-to-state transitions and their relative frequencies (max red scale = 0.01 transitions/second), originated from the idealization of individual FRET traces in FRET histograms.
(D) A modified spike (S383C and D985C) (Henderson et al., 2020; McCallum et al., 2020) stabilized in RBD-down conformation, observed from the FRET histogram (upper panel). The small increase in the population of the ground state (~0.5 FRET) likely reflects the partial nature of the formation of the disulfide in this mutant, which has 40% the infectivity of wild-type (Figure S3C). Modified S383C and D985C depicted in the high-resolution structure of S 6ZOY (lower panel).
(E–G) Experiments as in (A)–(C), respectively, conducted in the presence of 200 μg/mL monomeric hACE2. The soluble hACE2 activates spike proteins on the virus by shaping the conformational landscape toward stabilizing the RBD-up conformation (activated state). FRET histograms represent mean ± SEM, determined from three randomly assigned populations of all FRET traces under corresponding experimental conditions. N, number of individual FRET traces. Evaluated state occupancies see Table S1.
(H) Relative free-energy model of conformational landscapes of SARS-CoV-2 spikes in response to the hACE2 binding. The differences in free energies between states were roughly scaled based upon relative state occupancies of each state.