Figure 6.
Conceptualization of the Observed FRET States within Current High-Resolution Structures
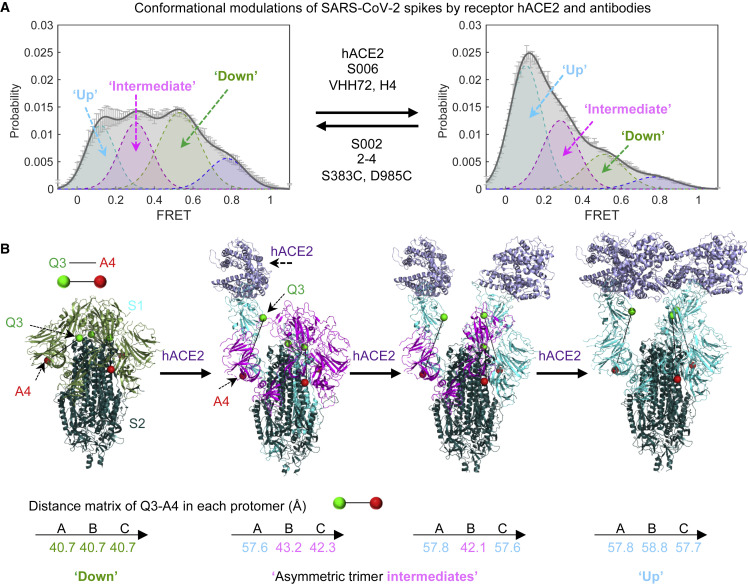
(A) Modulation of the conformational landscape observed by smFRET upon binding of receptor hACE2 or antibodies. The four conformational states are 0.1-FRET (dashed cyan), 0.3-FRET (dashed red), 0.5-FRET (dashed green), and 0.8-FRET (dashed magenta). smFRET histograms for ligand-free S and hACE2-bound are from Figures 2B and 2F, respectively.
(B) Interpretation of three of the observed smFRET states within the framework of cryo-EM structures of a soluble trimeric SARS-CoV-2 S, with GSAS and PP mutations and the T4 phage fibritin trimerization domain (Wrapp et al., 2020b). Ligand-free (PDB: 6VXX) and recognizing single, double, and triple ACE2 receptors molecules (Zhou et al., 2020), showing the distances between Q3-1 and A4-1 insertion sites on S1 in each protomer. The same color code is applied for conformational states within the smFRET histogram, individual S1 protomer in each cryo-EM structure and the distances between the fluorophores in each protomer of the spike trimer. See Discussion for details.