Fig. 1.
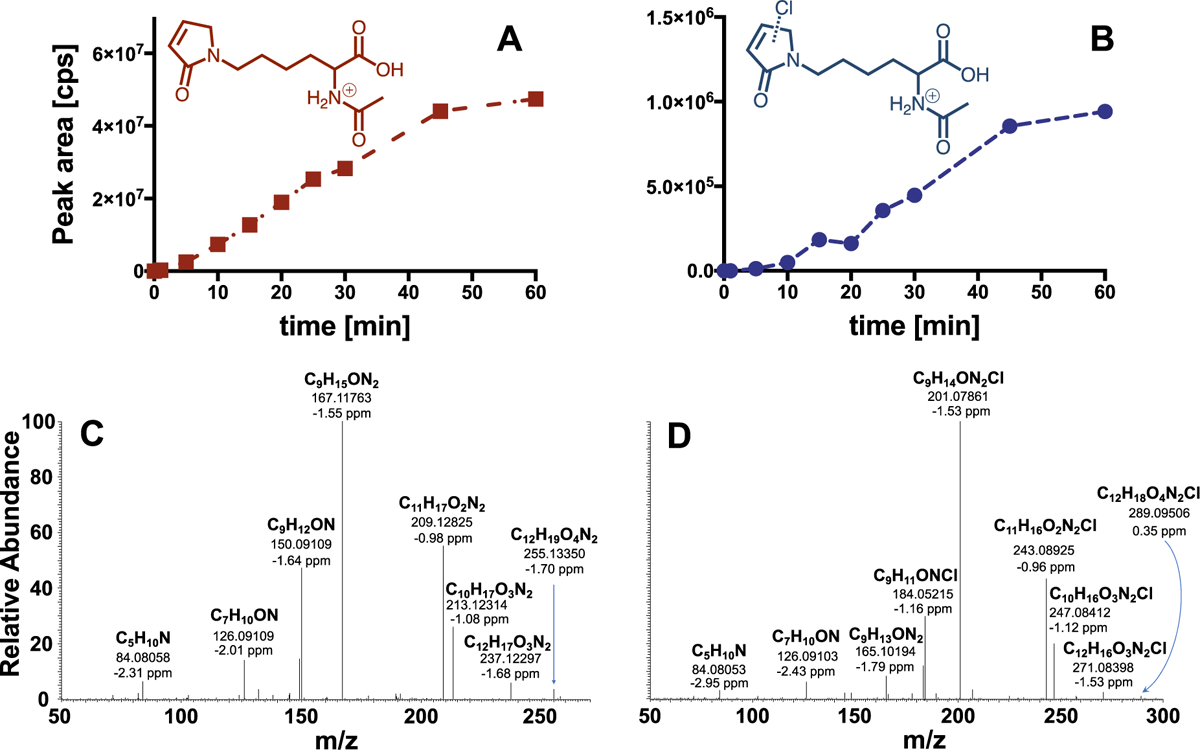
Kinetics of the formation of NAL-adducts (A) m/z 255 and (B) m/z 289 during reaction of phenol with chlorine (molar HOCl:phenol ratio 10:1; HOCl: 1 mM; phenol 0.1 mM). LC/MS/MS in precursor ion scan mode was used for identification of adducts while the chemical structures of the adducts were derived from HRMS analysis. Results of MS2 experiments with (C) m/z 255 and (D) m/z 289 show cleavage of H2O, cleavage of C2H2O and H2CO2. In addition, cleavage of NH3 from m/z 201 and 165 in MS2 spectrum of m/z 289 yielding m/z 184 and 148, respectively (i.e., before and after HCl elimination), and from m/z 167 in MS2 spectrum of m/z 255 yielding m/z 150 was observed. See SI for Cl isotope pattern observed for m/z 289 (Fig. S2).
