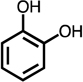
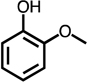
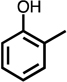
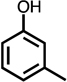
Table 1.
Formation of C4-dicarbonyl compounds during chlorination of various substituted phenols and non-phenolic aromatic compounds.
| C4-dialdehyde | catechol | guaiacol | o-cresol | m-cresol | p-cresol | 2,3-CH3-phenol | p-OH-benzoic acid | |
|---|---|---|---|---|---|---|---|---|
 |
 |
 |
 |
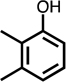 |
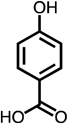 |
|||
| BDA | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | ✓ | |
| Cl-BDA | 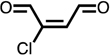 |
✓ | ✓ | ✕ | ✕ | ✕ | ✕ | ✓ |
| methyl-BDA | ✕ | ✕ | ✓ | ✓ | ✓ | ✕ | ✕ | |
| dimethyl-BDA | ✕ | ✕ | ✕ | ✕ | ✕ | ✓ | ✕ | |
| methyl-Cl-BDA | 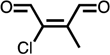 |
✕ | ✕ | ✕ | ✓ | ✕ | ✕ | ✕ |
|
Monoaromatic and phenolic compounds for which no C4- or methyl-C4-dicarbonyl formation was observed: | ||||||||
| benzene | toluene | Cl-benzene | p-CH3-anisole | 2,6-CH3-phenol | 2,4,6-CH3-phenol | resorcinol | hydroquinone | |
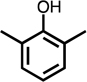 |
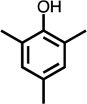 |
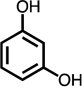 |
||||||
